Collaboration Delivers Robust eLearning Curriculum to Healthcare Schools and Hospitals to Elevate the Standard of Peripheral IV Access and Care BETHLEHEM, Pa., Sept. 29, 2022 /PRNewswire/ — Today B. Braun Medical Inc. (B. Braun), a leader in smart infusion therapy and pain management, together with the Association for Vascular Access (AVA), announced the launch […]
Tag: B Braun
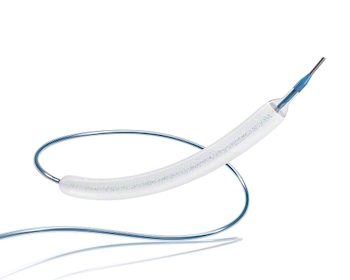
B. Braun Interventional Systems Inc., an Affiliate of B. Braun Medical Inc., and Infraredx, a Nipro Company, Announce Strategic U.S. Collaboration to Execute the IDE Clinical Trial for the SeQuent® Please ReX™ Drug Coated PTCA Balloon Catheter
Following Receipt of FDA Breakthrough Device Designation, Market Leading Global Organizations Strengthen their Cooperation to Navigate U.S. Regulatory Pathway BETHLEHEM, Pa., March 31, 2021 /PRNewswire/ — B. Braun Interventional Systems Inc. (BIS) announced today that they will collaborate with Infraredx, a Nipro Company, to accelerate the U.S. Food and Drug Administration (FDA) Investigational Device Exemption […]
B. Braun Interventional Systems Expands its Congenital and Structural Heart Portfolio With the EmeryGlide™ MR Conditional Guidewire From Nano4Imaging
BETHLEHEM, Pa., Aug. 29, 2019 (GLOBE NEWSWIRE) — B. Braun Interventional Systems Inc. (BIS), a company dedicated to providing innovative solutions in the field of congenital and structural interventional cardiology, today announced it has signed an exclusive U.S. distribution agreement with Nano4imaging for their EmeryGlide™ guidewire. The EmeryGlide MR conditional […]
B. Braun Interventional Systems Receives Breakthrough Device Designation Status from the FDA for SeQuent Please ReX Drug Coated PTCA Balloon Catheter
BETHLEHEM, Pa., Aug. 01, 2019 (GLOBE NEWSWIRE) — B. Braun Interventional Systems Inc. (BIS) announced today that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the SeQuent Please ReX drug-coated PTCA balloon catheter for the treatment of coronary in-stent restenosis (ISR). SeQuent Please ReX is […]
B. Braun to Showcase Its Commitment to Reducing Healthcare-Associated Infections with Products and Education at APIC 2018
BETHLEHEM, Pa., June 08, 2018 (GLOBE NEWSWIRE) — B. Braun Medical Inc. will feature products that help fight healthcare-associated infections – and will support (through an unrestricted educational grant to Saxe Healthcare Communications) a continuing education (CE) accredited presentation about a new technique that can be used to proactively address […]