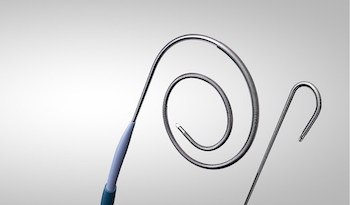
MISSISSAUGA, ON, June 5, 2024 /PRNewswire/ – Baylis Medical Technologies today announced the completion of its first clinical use of the PowerWire® Pro Radiofrequency (RF) Guidewire to safely cross a chronically occluded peripheral stent.
Continue Reading
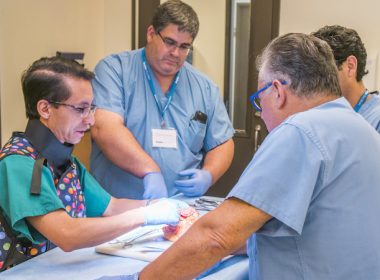
Photograph of Dr. Iafrati and his team holding up a PowerWire® Pro box after their successful case. (CNW Group/Baylis Medical Technologies Inc.)
The procedure was performed in April 2024, by Mark Iafrati, MD, Professor of Vascular Surgery in Nashville, TN. The patient was suffering from a chronically occluded stent that extended from the inferior vena cava (IVC) down to the left common iliac vein. Previous attempts to cross the occlusion with mechanical tools were unsuccessful, likely owing to the dense fibrotic nature of the occlusion. The patient was brought back and Dr. Iafrati performed the procedure with the PowerWire Pro RF Guidewire, which utilizes RF energy to vaporize a channel through occlusions, allowing for successful crossing and subsequent revascularization of the iliocaval stent.
“This marks a significant advancement in the treatment of patients with in-stent restenosis”, commented Dr. Iafrati. “I was able to easily cross the occluded stents with the PowerWire Pro RF Guidewire when my standard tools had failed.”
This spring, the FDA cleared Baylis Medical Technologies’ PowerWire Pro RF Guidewire, a device designed to cross occluded peripheral vessels, including those with stents, using radiofrequency technology.Frank Baylis, Executive Chairman of Baylis Medical Technologies, expressed enthusiasm about the PowerWire® Pro RF Guidewire’s potential impact, stating, “This innovation aims to simplify the often challenging and time-consuming process of crossing chronic occlusions in revascularization procedures, ensuring uninterrupted patient treatment.”
About Baylis Medical Technologies Inc.
Baylis Medical Technologies seeks to improve the lives of patients through the conception and commercialization of state-of-the-art medical devices. The company proudly carries forward Gloria Baylis’ legacy to enhance access to care through its divisions of Endovascular and Design and Manufacturing Services. Baylis Medical Technologies’ clinical solutions are utilized by healthcare professionals worldwide to improve patient outcomes for individuals with cardiovascular and other medical conditions. Learn more about our innovative products and ongoing mission at www.baylismedtech.com.For any inquiries, please contact [email protected] or call 1-888-505-4885
PRM-00910 J-1 V-1 © Baylis Medical Technologies Inc., 2024. PowerWire is a trademark and/or registered trademark of Baylis Medical Technologies Inc. in the United States and other countries. Baylis Medical Technologies Inc. reserves the right to change specifications or to incorporate design changes without notice and without incurring any obligation relating to equipment previously manufactured or delivered. Patents Pending and/or issued. CAUTION: Federal Law (USA) restricts the sale of these devices to or by the order of a physician. Before use, consult product labels and inserts for any indications, contraindications, hazards, warnings, cautions and instructions for use.
SOURCE Baylis Medical Technologies Inc.