SAN CARLOS, Calif.–(BUSINESS WIRE)–BioCardia, Inc. [OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced that the FDA has approved an Investigational Device Exemption for the CardiAMP Chronic Myocardial Ischemia (CMI) Trial to treat patients with refractory angina (RA). The CardiAMP Chronic Myocardial Ischemia Trial will be […]
Tag: BioCardia
BioCardia Submits Clinical Trial to FDA for New Indication of Chronic Myocardial Ischemia for CardiAMP Cell Therapy
SAN CARLOS, Calif.–(BUSINESS WIRE)-–BioCardia, Inc. [OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today reported filing a second Investigational Device Exemption with the FDA for the CardiAMP Chronic Myocardial Ischemia Trial to treat patients with refractory angina. This second potential indication for CardiAMP investigational cell therapy of […]
BioCardia Announces Reverse Stock Split and Release of Indemnity Holdback Shares
SAN CARLOS, Calif.–(BUSINESS WIRE)– BioCardia, Inc.[OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced a reverse split of its common stock, $0.001 par value, at a ratio of 1 for 12, effective November 2, 2017 (the “Effective Date”). The company’s common stock will begin […]
BioCardia Receives U.S. Patent Covering Morph Product Family Design
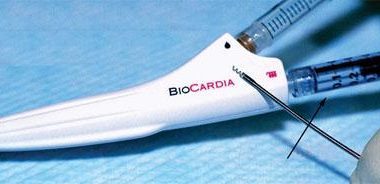
SAN CARLOS, Calif.–(BUSINESS WIRE)–BioCardia®, Inc. (OTC: BCDA), a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, with clinical programs in heart failure, today announced the issuance of United States Patent No. 9,775,963 entitled, “Steerable Endoluminal Devices and Methods.” BioCardia CEO Peter Altman, PhD stated, “This new patent […]
BioCardia Announces Successful Interim Safety Analysis In Its Phase III Clinical Trial Of Cardiamp Stem Cell Therapy For Heart Failure
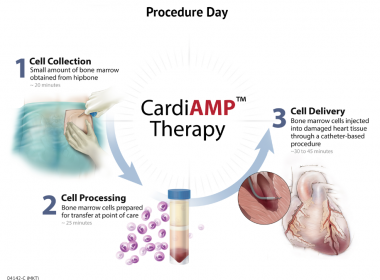
SAN CARLOS, Calif.–(BUSINESS WIRE)–BioCardia (OTC: BCDA), a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced that the independent Data Safety Monitoring Board (DSMB) has completed the pre-specified interim analysis of safety outcomes for the first 10 patients treated in the Phase 3 trial of its […]
BioCardia Announces Publication Of 12-Month Results From Phase II TRIDENT Clinical Trial That Shows Positive Safety Profile For Allogeneic Bone Marrow Stem Cells Delivered With Helix Transendocardial Delivery System
DALLAS & SAN CARLOS, Calif.–(BUSINESS WIRE)–BioCardia®, Inc. [OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced 12-month results from the Phase II TRIDENT clinical trial, conducted by the University of Miami Miller School of Medicine and co-sponsored by the company. The results showed a […]
BioCardia’s CardiAMP Heart Failure Trial Design To Be Presented At Texas Heart Institute International Symposium On Cardiovascular Regenerative Medicine
HOUSTON & SAN CARLOS, Calif.–(BUSINESS WIRE)–BioCardia®, Inc. [OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced that the trial design for its pivotal Phase III CardiAMP Heart Failure Trial will be presented tomorrow during the “Heart Failure: The Big Target for CV Regenerative Therapy […]
BioCardia Receives U.S. Patent Covering A Method Of Preparing And Characterizing Mesenchymal Cells, Providing Further Protection To Cardiallo Cell Therapy Program
SAN CARLOS, Calif.–(BUSINESS WIRE)–BioCardia®, Inc. [OTC:BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, with clinical programs in heart failure and sub-acute infarction, today announced the issuance of United States Patent No. 9,301,975 relating to a method of producing mesenchymal stem cells from bone marrow cells in […]
BioCardia Completes Roll-In Cohort In Pivotal Phase III CardiAMP Heart Failure Trial
SAN CARLOS, Calif.–(BUSINESS WIRE)–BioCardia®, Inc. [OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced completion of treatment for the 10-patient roll-in cohort for the pivotal Phase III CardiAMP Heart Failure Trial. A pre-specified review of the 30-day outcomes in this cohort by the Data […]