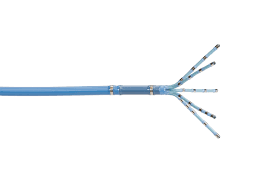
IRVINE, Calif., July 24, 2023 /PRNewswire/ — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & Johnson MedTech,i today announced the U.S. launch of the OPTRELL™ Mapping Catheter with TRUEref™ Technology powered by the CARTO® 3 System. The OPTRELL™ Mapping Catheter is a high-density diagnostic catheter, with small […]
Tag: Biosense
With Innovative Health’s landmark clearance to reprocess the leading electrophysiology mapping catheter from Biosense Webster, single-use device reprocessing has entered a new era.
SCOTTSDALE, Ariz., June 28, 2019 /PRNewswire-PRWeb/ — Innovative Health, the leading single-use cardiology medical device reprocessing company, today announced it has received FDA clearance for reprocessing the market-leading PENTARAY® Nav eco High-Density mapping catheter (hereinafter PentaRay), a development that represents both a technological and healthcare cost reduction milestone. The PentaRay is a key […]
BIOSENSE WEBSTER ANNOUNCES INITIAL RESULTS FROM FIRST IN-HUMAN STUDY OF NOVEL HIGH POWER-SHORT DURATION ABLATION CATHETER FOR ATRIAL FIBRILLATION
Researchers Report QDOT MICRO Radiofrequency Ablation Catheter Demonstrates Safety and Achieves Pulmonary Vein Isolation with Shorter Procedure and Fluoroscopy Times SAN FRANCISCO — May 9, 2019 – Johnson & Johnson Medical Devices Companies* today announced that Biosense Webster, Inc.’s QDOT MICRO, a novel catheter that facilitates high power-short duration radiofrequency […]
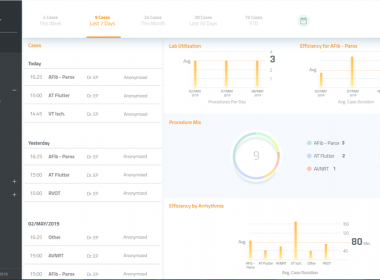
INTELLIGENT DATA ANALYTICS SOFTWARE SOLUTION COMES TO ELECTROPHYSIOLOGY WITH LAUNCH OF BIOSENSE WEBSTER’S CARTONET
San Francisco– May 7, 2019 – Johnson & Johnson Medical Devices Companies* today announced the launch of Biosense Webster, Inc.’s CARTONET as part of the company’s mission to help revolutionize the way electrophysiologists, hospitals, health systems, and researchers leverage and share data with the goal of improving patient outcomes and […]
First Atrial Fibrillation Patient Treated In Biosense Webster U.S. IDE Study Evaluating High Power, Short Duration Ablation Catheter
IRVINE, Calif., Feb. 4, 2019 /PRNewswire/ — Johnson & Johnson Medical Devices Companies* announced today that Biosense Webster, Inc., a worldwide leader in the diagnosis and treatment of heart arrhythmias, has enrolled and treated the first patient in its U.S. Investigational Device Exemption (IDE) study** which evaluates the company’s QDOT MICRO Radiofrequency […]
Biosense Webster, Inc. Launches Study To Evaluate Novel High Power, Short Duration Modality For Treatment Of Atrial Fibrillation
IRVINE, Calif., May 2, 2018 /PRNewswire/ — Johnson & Johnson Medical Devices Companies* announced today that Biosense Webster, Inc., a worldwide leader in the diagnosis and treatment of heart arrhythmias, has enrolled and treated the first patient in its QDOT AF Study.** The study will evaluate the delivery of high power, short duration ablation […]