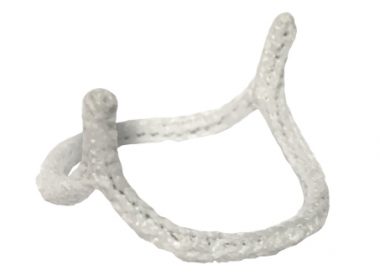
First commercial implantations of the HAART 200 Device for Bicuspid Aortic Valve repair performed by Professor Marek Jasinski at the Wroclaw Medical University AUSTIN, Texas–(BUSINESS WIRE)–BioStable Science & Engineering, Inc. announced today that the first commercial implantations of its HAART 200 Aortic Annuloplasty Device were performed on Monday, August 2nd by Professor […]
Tag: BioStable Science & Engineering
BioStable Science & Engineering Announces CE Mark Approval for the HAART 200 Aortic Annuloplasty Device & Surpasses 1,000 Patients Treated Worldwide with the HAART Devices
AUSTIN, Texas–(BUSINESS WIRE)–BioStable Science & Engineering, Inc. (“BioStable”) announced today the company has received CE Mark approval for the HAART 200 Aortic Annuloplasty Device for use during bicuspid aortic valve repair. With CE Mark approval of both the HAART 300 and HAART 200 Aortic Annuloplasty Devices, BioStable will be able to […]
BioStable Science & Engineering Announces the First Commercial Use of the HAART 200™ Aortic Annuloplasty Device
AUSTIN, Texas–(BUSINESS WIRE)–BioStable Science & Engineering, Inc. announced today the first commercial use of the HAART 200 Aortic Annuloplasty Device in the U.S. by doctors at the West Virginia University (WVU) Heart and Vascular Institute in Morgantown, West Virginia. Drs. Lawrence Wei, Vinay Badhwar and J. Scott Rankin were the […]