WAYNE, Pa., July 01, 2025 (GLOBE NEWSWIRE) — Teleflex Incorporated (NYSE:TFX), a leading global provider of medical technologies, today announced that it has completed the previously announced acquisition of substantially all of the Vascular Intervention business of BIOTRONIK SE & Co. KG. The acquisition adds a broad portfolio of therapeutic products to Teleflex’s portfolio of interventional access products, driving an enhanced global presence in the cath lab. The Vascular Intervention business will also establish Teleflex’s global footprint in the fast-growing peripheral intervention market, and provide a channel for Teleflex products that currently have a peripheral indication.
Tag: Biotronik
Teleflex to Acquire BIOTRONIK’s Vascular Intervention Business
Acquisition will further advance Teleflex’s Interventional portfolio with a differentiated global suite of coronary vascular and peripheral vascular intervention devices Acquisition will further advance Teleflex’s Interventional portfolio with a differentiated global suite of coronary vascular and peripheral vascular intervention devices
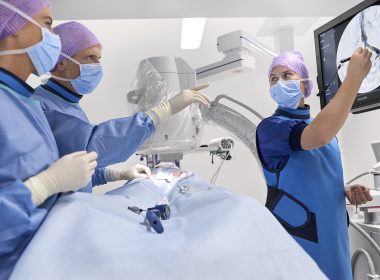
BIOTRONIK and Egg Medical Form Strategic Alliance to Improve Radiation Protection for Interventional Healthcare Workers
BIOTRONIK, a global leader in cardiovascular, endovascular, and neuromodulation solutions, today announced it is partnering with Egg Medical, Inc., a global leader in radiation protection technology, to co-sell Egg Medical’s EggNest™ Radiation Protection Systems for healthcare workers in the United States. The EggNest Systems (EggNest XR, EggNest Protect & EggNest Complete) are […]
BIOTRONIK, INC. Announces First US Implant of Next-Generation Amvia Edge Pacemaker
LAKE OSWEGO, Ore., Aug. 29, 2023 /PRNewswire/ — BIOTRONIK, a leader in implantable medical device technology, today announced its first US implantation of the new Amvia Edge pacemaker. The procedure was successfully completed by Dr. Raul Weiss at Mount Sinai Medical Center in Miami, Florida. Amvia Edge is BIOTRONIK’s next-generation pacemaker family, featuring MRI Guard 24/7, […]
BIOTRONIK Receives FDA Approval For Next-Generation Family of Pacemakers
The Amvia Edge pacemaker platform introduces always-on, automatic MR detection algorithm to fully streamline MRI workflow LAKE OSWEGO, Ore., July 6, 2023 /PRNewswire/ — BIOTRONIK today announced U.S. Food and Drug Administration (FDA) approval of its portfolio of Amvia Edge pacemakers and cardiac resynchronization therapy pacemaker (CRT-P), its latest innovation in cardiac rhythm management. Amvia Edge, […]
BIOTRONIK Demonstrates Ongoing Commitment to Advancing Scientific Knowledge at HRS
Four studies shown at Heart Rhythm 2023 were generated from BIOTRONIK’s CERTITUDE research program LAKE OSWEGO, Ore., June 23, 2023 /PRNewswire/ — Continuing a history of commitment to furthering research in its field, four studies supported by BIOTRONIK‘s real-world evidence generating research initiative, CERTITUDE, were presented at Heart Rhythm 2023 on May 20th and 21st. The […]
Philips and BIOTRONIK expand care for out-of-hospital cardiology labs with new strategic alliance
Alliance further strengthens Philips’ industry-leading SymphonySuite solution, a complete, integrated offering to support clinicians or hospitals to open and operate office-based labs and ambulatory surgery centers Addition of BIOTRONIK’s cardiovascular technologies and products to the Philips SymphonySuite device programs expands the volume of procedures clinicians can perform in out-of-hospital settings, […]
BIOTRONIK ANNOUNCED FIRST IMPLANT OF NEW IMPLANTABLE CARDIAC MONITOR WITH ARTIFICIAL INTELLIGENCE, TARGETING EFFICIENCY IN MONITORING FOR CARDIAC ARRHYTHMIAS
Montefiore Medical Center in New York performed the first global implantation of the new BIOMONITOR IV ICM LAKE OSWEGO, Ore., June 12, 2023 /PRNewswire/ — BIOTRONIK, a leader in implantable medical device technology, today announced the first global implantation of its BIOMONITOR IV implantable cardiac monitor (ICM). The procedure was conducted by Luigi Di Biase, MD, […]
BIOTRONIK Enrolls First Patient Into BIO-CONDUCT, First IDE Trial to Study Use of Stylet-Driven Leads for Conduction System Pacing
LAKE OSWEGO, Ore., Dec. 12, 2022 /PRNewswire/ — BIOTRONIK, a global leader in the treatment of cardiac and vascular disease is pleased to announce the first patient enrollment in BIO-CONDUCT, an FDA approved investigational device exemption (IDE) trial examining the use of the BIOTRONIK Solia S pacing lead when implanted in the […]
BIOTRONIK Announces Collaboration with AliveCor As First In A Series of Strategic Partnerships to Disrupt Cardiac Digital Health With AI-Enabled Solutions
Designed to Leverage AI Technology to Improve the Delivery of Connected Care in Cardiac Diagnostics and Remote Patient Monitoring LAKE OSWEGO, Ore., Sept. 8, 2022 /PRNewswire/ — BIOTRONIK announced today its partnership with AliveCor, a leading innovator in FDA-cleared personal electrocardiogram (ECG) technology and services. This partnership will combine the strengths of BIOTRONIK’s BIOMONITOR Injectable […]