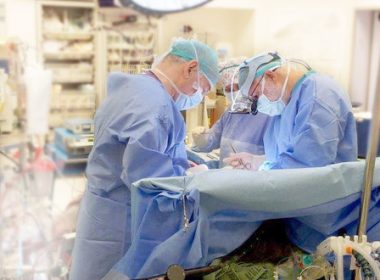
LAKE OSWEGO, Ore., Aug. 31, 2022 /PRNewswire/ — BIOTRONIK’s LiveSupport feature has been successfully used to remotely support Cardiac Rhythm Management (CRM) clinics. This new technology enables BIOTRONIK representatives to provide programmer support from remote locations, allowing for faster support for clinics and addressing concerns around in-person contact brought on by the […]
Tag: Biotronik
First and Only Peripheral Tri-axial 4-French Low-Profile Self-Expanding Stent System Receives FDA Approval
BIOTRONIK Launches its Pulsar-18 T3 Self-Expanding Stent System in the U.S. LAKE OSWEGO, Ore., July 26, 2022 /PRNewswire/ — BIOTRONIK, LAKE OSWEGO, USA, announced that it received U.S. Food and Drug Administration (FDA) approval of its innovative Pulsar®-18 T3 peripheral self-expanding stent system for an improved implantation procedure for endovascular treatments. The company also announced the full U.S. commercial […]
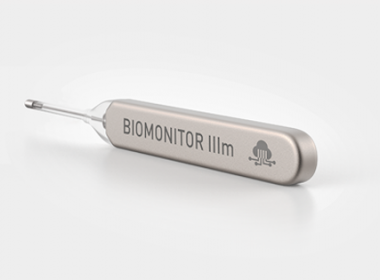
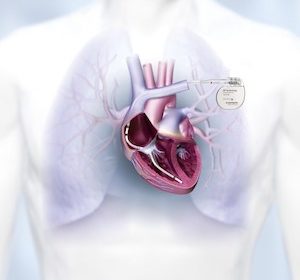
BIOTRONIK’s State-of-the-Art LiveSupport Successfully Used in BIOMONITOR Implantable Cardiac Monitor Implants
LAKE OSWEGO, Ore., July 6, 2022 /PRNewswire/ — BIOTRONIK’s state-of-the-art LiveSupport feature was used to remotely support two BIOMONITOR Implantable Cardiac Monitor (ICM) implants shortly after launch. Both procedures were performed with a BIOTRONIK representative in a remote location. The physicians are pleased with the results and the support offered; they are […]
BIOTRONIK Announces FDA Approval of Renamic Neo with LiveSupport Software
LAKE OSWEGO, Ore., April 26, 2022 /PRNewswire/ — BIOTRONIK, Inc. announced today it has received U.S. Food and Drug Administration (FDA) approval of Renamic Neo, the company’s state-of-the-art programmer for implanted cardiac rhythm management devices such as ICDs, pacemakers, and implantable cardiac monitors. In addition to its programming functions, Renamic Neo now […]
BIOTRONIK Sets New Clinical Benchmark with Ultrathin Strut Orsiro Coronary Drug-Eluting Stent
Final 5-Year Data from BIOFLOW-V Trial Confirms Better Safety for Orsiro DES, Confirming Orsiro’s Position as the Clinical Benchmark for DES BÜLACH, Switzerland, Feb. 28, 2022 /PRNewswire/ — BIOTRONIK is pleased to announce five-year data from the BIOFLOW-V trial, which was presented yesterday at the 2022 CRT Conference during a late-breaking clinical trial session by Dr. David […]
FDA Approval and First Implant of Orsiro Mission Drug-Eluting Stent System in the U.S.
Full Commercial Launch Underway for One of the Most Studied and Thinnest Strut Drug-Eluting Stents, Offering Enhanced Deliverability LAKE OSWEGO, Ore., Sept. 29, 2021 /PRNewswire/ — BIOTRONIK announced today it received U.S. Food and Drug Administration (FDA) approval of its Orsiro® Mission bioabsorbable polymer drug-eluting stent system (BP-DES). The company also announced the first U.S. […]
First Real-World Data on the Utility of Device-derived Daily Activity, a Novel Digital Biomarker, to Predict Ventricular Arrhythmias
Data from the CERTITUDE Registry Presented at ESC 2021 LAKE OSWEGO, Ore., Sept. 9, 2021 /PRNewswire/ — Following the European Society of Cardiology (ESC) Congress 2021, BIOTRONIK today announced the first observational results of the CERTITUDE registry. The registry is designed to evaluate real-world clinical outcomes of patients in the United States who use implantable […]
Latest Study Shows New Algorithm Can Predict Heart Failure Hospitalizations
SELENE HF adds to existing evidence that HF decompensation starts several weeks before hospitalization BERLIN, Aug. 18, 2021 /PRNewswire/ — BIOTRONIK today announced the results from a new study published in EUROPACE this week confirming that heart failure (HF) decompensation can be predicted early when monitored using an algorithm that combines existing remote monitoring trends and baseline […]
New Study Aims to Show Effectiveness of CRT-D Technology for Heart Failure Patients with Atrial Fibrillation
First Enrollments Underway for BIO-AffectDX Trial Evaluating Advanced, Two-Lead CRT-DX System LAKE OSWEGO, Ore., July 30, 2021 /PRNewswire/ — In association with Heart Rhythm 2021, BIOTRONIK today announced first enrollments in the landmark BIO-AffectDX trial, designed to evaluate clinical outcomes of cardiac resynchronization therapy (CRT) in patients with heart failure (HF) and atrial fibrillation (AF). BIO-AffectDX is a multi-center, observational study that will enroll HF […]
BIOTRONIK’s Implantable Cardiac Monitor Receives Prestigious Industry Award
BIOMONITOR IIIm Received Frost & Sullivan’s ‘Enabling Technology Leadership Award’ 2021 for its High Precision Implantable Cardiac Monitoring BERLIN, July 28, 2021 /PRNewswire/ — In association with Heart Rhythm 2021, BIOTRONIK today announced that the latest implantable cardiac monitor (ICM), BIOMONITOR IIIm, has been recognized by internationally-renowned research analysts Frost & Sullivan as […]