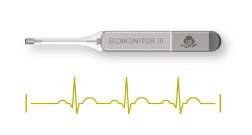
BERLIN–(BUSINESS WIRE)–BIOTRONIK today announced the market release of its new injectable cardiac monitor (ICM), BIOMONITOR III, following approval in the CE region. The novel device is designed to help patients with irregular heart rhythms by documenting suspected arrhythmia or unexplained syncope with increased clarity. As the most common type of arrhythmia, 33.5 […]
Tag: Biotronik
Focusing on Women: BIOTRONIK Launches Study to Understand the Impact of ICDs in NICM Patients
LAKE OSWEGO, Ore., May 6, 2019 /PRNewswire/ — BIOTRONIK today announced initial enrollment into the BIO-LIBRA study, a first-of-its kind, large-scale prospective study to analyze sex-specific outcomes in patients with non-ischemic cardiomyopathy (NICM) treated with cardiac defibrillator device therapy. To adequately analyze whether women and men respond differently to defibrillator therapy, a minimum of […]
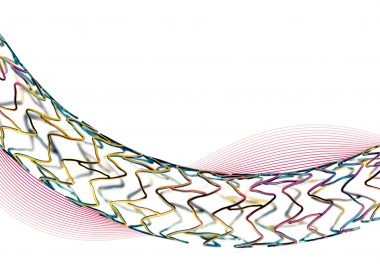
BIOTRONIK Launches PK Papyrus Covered Coronary Stent in the US
LAKE OSWEGO, Ore., April 10, 2019 /PRNewswire/ — BIOTRONIK today announced the United States (US) commercial launch of the PK Papyrus® covered coronary stent system for use in the emergency treatment of acute coronary perforations.1,2 More than 800,000 percutaneous coronary intervention procedures are performed annually in the US3 and fewer than 8,000 require a covered stent, classifying PK […]
FDA Approves Industry’s Smallest, Slimmest 3T Tachycardia Devices from BIOTRONIK
LAKE OSWEGO, Ore., March 14, 2019 /PRNewswire/ — BIOTRONIK today announced FDA approval of the Acticor and Rivacor high-voltage cardiac rhythm management (CRM) device families for treatment of patients with cardiac arrhythmias. The six new tachycardia solutions include Rivacor VR-T, Rivacor DR-T, Rivacor HF-T QP, Acticor DX, Acticor CRT-DX Bipolar and Acticor […]
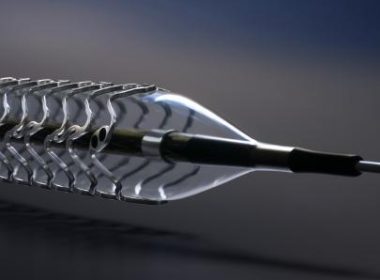
TCT Congress 2018: BIOTRONIK’s Orsiro Ultrathin Drug-Eluting Stent Further Outperforms Xience at Two-Year Follow-Up
SAN DIEGO and BUELACH, Switzerland, Sept. 24, 2018 /PRNewswire/ — Today, BIOTRONIK announced two-year data from the BIOFLOW-V randomized trial comparing the Orsiroa and Xienceb drug-eluting stents (DES). The data shows statistically improved outcomes for patients treated with Orsiro across a range of clinical endpoints including 24-month target lesion failure (TLF). This pivotal US investigational device exemption trial included […]
InfoBionic Signs BIOTRONIK as Exclusive U.S. Distributor for MoMe® Kardia
BOSTON–(BUSINESS WIRE)–InfoBionic, a digital health company focused on creating next-generation solutions for ambulatory, cardiac arrhythmia monitoring, today announced an exclusive, strategic agreement with medical device manufacturer BIOTRONIK for U.S. distribution of InfoBionic’s proprietary MoMe® Kardia system, effective immediately. “BIOTRONIK is a trusted technology provider throughout the healthcare industry and we believe that […]
BIOTRONIK Advances Arrhythmia Detection and Diagnosis with MoMe Cardiac Monitor in the US
LAKE OSWEGO, Ore., Aug. 27, 2018 /PRNewswire/ — BIOTRONIK today announced that the company is now the exclusive US distributor for InfoBionic’s MoMe Kardia external cardiac diagnostic monitor. The device benefits patients suspected of experiencing cardiac arrhythmias. Patients experiencing cardiac arrhythmias face an increased risk of personal injury from falls, motor vehicle accidents, stroke […]
Improving Cardiology Care: BIOTRONIK US to Distribute Aziyo ECM Envelopes
LAKE OSWEGO, Ore. and SILVER SPRING, Md., April 3, 2018 /PRNewswire/ — BIOTRONIK US and Aziyo today announced a strategic agreement allowing BIOTRONIK to distribute Aziyo’s CanGaroo® extracellular matrix (ECM) cardiovascular implantable electronic device (CIED) envelopes in the United States. BioEnvelope will be available from BIOTRONIK starting in April 2018. BioEnvelope reduces scar formation and has been shown to mitigate the […]
TCT 2017: BIOTRONIK Symposium Highlights Differing Roles of Drug-Eluting Stents and Magnesium Scaffolds in Clinical Practice
Denver, United States / Buelach, Switzerland, 31.10.2017 (PresseBox) – Data presented at the BIOTRONIK-sponsored symposium on the Orsiro1 drug-eluting stent (DES) demonstrate why Orsiro is gaining prominence in the crowded DES market. In addition, clinical and preclinical data presented about Magmaris2 reinforce the case that the magnesium-based resorbable technology does not share the same risk of […]
BIOTRONIK Announces First Enrollments to BIOVITESSE Trial
(PresseBox) – BIOTRONIK has announced the start of enrollment of a coronary stent trial aiming at assessing the safety and clinical performance of a new coronary stent in de novo coronary artery lesions. On September 28, first Dr. Marco Moccetti, Cardiocentro Ticino, Lugano, Switzerland, and later on the same day Dr. Lorenz Raeber, University […]