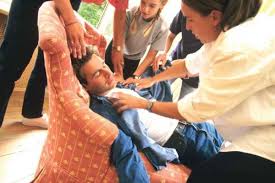
Robustly Positive Results of SPAIN Study Published in Journal of American College of Cardiology BERLIN, Germany, October 4, 2017 – BIOTRONIK’s exclusive rate responsive technology, Closed Loop Stimulation (CLS) has shown to reduce fainting in pacemaker patients with vasovagal syncope up to sevenfold. Results from the SPAIN study, the first […]
Tag: Biotronik
ESC Congress 2017: BIOTRONIK’s Orsiro Drug-Eluting Stent Outperforms Xience in BIOFLOW-V Trial
Landmark Data Published by The Lancet Demonstrates Statistically Significant Lower Event Rates with Orsiro BARCELONA, Spain, August 28, 2017 – BIOTRONIK today announced data from the BIOFLOW-V randomized trial comparing Orsiro1 and Xience2 drug-eluting stents (DES) with 12-month target lesion failure (TLF) as the primary endpoint proving non-inferiority. Results presented at the […]
BIOTRONIK US Launches Smallest MR Conditional Quadripolar Cardiac Resynchronization Therapy Pacemaker
LAKE OSWEGO, Ore., Aug. 21, 2017 /PRNewswire/ — BIOTRONIK today announced FDA approval and commercial availability of Edora HF-T QP, an MR conditional quadripolar (QP) cardiac resynchronization therapy pacemaker (CRT-P) with MRI AutoDetect technology. With a volume of 15 cc, Edora HF-T QP is the smallest MR conditional CRT-P available in the US with […]
Fewer Leads, Fewer Complications: BIOTRONIK US Launches Proven DX Technology for Heart Failure Patients
LAKE OSWEGO, Ore., July 19, 2017 /PRNewswire/ — BIOTRONIK today announced FDA approval and availability of the Intica DX and Intica cardiac resynchronization therapy (CRT)-DX implantable cardioverter defibrillator (ICD) systems. The launch of Intica CRT-DX extends the proven benefits of BIOTRONIK’s DX technology to heart failure patients. DX eliminates the need for an atrial […]
BIOTRONIK’s New Quadripolar CRT-P with More Pacing Options Now Available in Japan
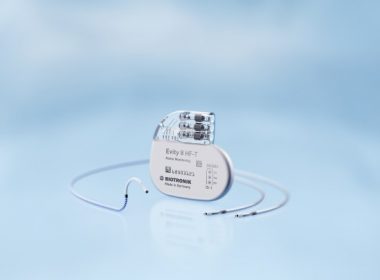
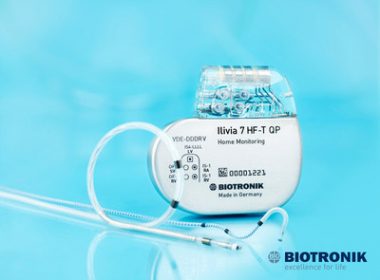
Evity 8 HF-T QP Offers a Host of Functions Including LV VectorOpt, RF Telemetry, MRI AutoDetect and BIOTRONIK Home Monitoring TOKYO, Japan and BERLIN, Germany, July 10, 2017 – BIOTRONIK has announced the launch of its Evity cardiac resynchronization therapy pacemaker (CRT-P) in the Japanese market. It is the company’s highest performing […]
BIOTRONIK US Announces Pivotal Trial Results of the Pulsar-18 Stent with 4-French Delivery System
NEWS PROVIDED BY BIOTRONIK Jul 03, 2017, 10:00 ET LAKE OSWEGO, Ore., July 3, 2017 /PRNewswire/ — BIOTRONIK today announced the results of the BIOFLEX-I clinical study and availability of Pulsar®-18 for the treatment of patients with peripheral artery disease (PAD). Pulsar-18 is the only superficial femoral artery (SFA) self-expanding stent approved by the FDA with […]
BIOTRONIK Launches Edora Pacemaker Series with MRI AutoDetect Technology
LAKE OSWEGO, Ore., June 7, 2017 /PRNewswire/ — BIOTRONIK today announced the availability of the Edora line of devices, the company’s first available pacemaker series featuring BIOTRONIK’s MRI AutoDetect technology. Edora SR-T is the smallest MR conditional pacemaker with automated MRI detection capability available in the US with a volume of […]
FDA Green Lights BIOTRONIK’s Sentus ProMRI Quadripolar Left Ventricular Lead
LAKE OSWEGO, Oregon, May 8, 2017 /PRNewswire/ — BIOTRONIK today announced FDA approval and the launch of Sentus ProMRI®, the thinnest quadripolar left ventricular lead available in the United States. This introduction completes BIOTRONIK’s second-generation ProMRI lead portfolio, which also includes Solia ProMRI and Plexa ProMRI. Sentus ProMRI is approved for […]
BIOTRONIK Nabs FDA OK of MultiPole Pacing with ProMRI: 360° Solutions for Patients With Heart Failure
LAKE OSWEGO, Ore., May 8, 2017 /PRNewswire/ — BIOTRONIK today announced FDA approval of the company’s MultiPole Pacing (MPP) technology, providing physicians with additional treatment options for heart failure patients who have been non-responsive to cardiac resynchronization therapy (CRT).1 MPP will be available on new BIOTRONIK CRT defibrillator (CRT-D) systems for […]
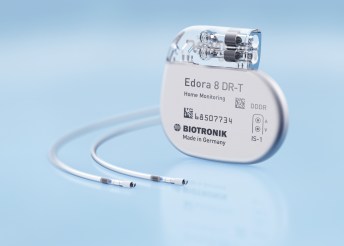
BIOTRONIK Launches Edora Pacemaker Series with MRI AutoDetect Technology
LAKE OSWEGO, Ore., June 7, 2017 /PRNewswire/ — BIOTRONIK today announced the availability of the Edora line of devices, the company’s first available pacemaker series featuring BIOTRONIK’s MRI AutoDetect technology. Edora SR-T is the smallest MR conditional pacemaker with automated MRI detection capability available in the US with a volume of […]