Acquisition to expand Boston Scientific’s cardiovascular portfolio and further address increasing prevalence of vascular diseases Provides scaled entry into mechanical thrombectomy and neurovascular, key strategic adjacencies Conference call at 8:00 a.m. ET to discuss details of the transaction MARLBOROUGH, Mass. and ALAMEDA, Calif., Jan. 15, 2026 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) and Penumbra, Inc., […]
Tag: Boston Scientific
The FARAPULSE™ Pulsed Field Ablation System Demonstrates Superior Efficacy in Patients Treated for Paroxysmal Atrial Fibrillation
New findings from the SINGLE SHOT CHAMPION trial (NCT05534581), presented at EHRA 2025 and published in the New England Journal of Medicine, showed the FARAPULSE™ Pulsed Field Ablation (PFA) System to be superior in reducing atrial arrhythmia (AA) recurrence for treating symptomatic, drug-refractory paroxysmal atrial fibrillation (PAF) compared to the Artic Front Advance™ cardiac cryoablation catheter. […]
Ajax Health and KKR Form New Platform to Develop System for Treating Heart Failure
MENLO PARK, Calif., March 4, 2025 /PRNewswire/ — Ajax Health, a KKR-backed medical device platform, today announced the formation of a new entity, FlowMod. The new organization is the result of a collaboration between Boston Scientific Corporation, Ajax Health, and KKR, utilizing…
Boston Scientific announces agreement to acquire SoniVie Ltd.
Acquisition to expand Interventional Cardiology Therapies offerings with ultrasound-based renal denervation therapy for treatment of hypertension MARLBOROUGH, Mass., March 3, 2025 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced it has entered into a definitive…
Late-breaking data presented at AF Symposium 2025 highlight key Boston Scientific therapies for management of patients with atrial fibrillation
First phase of ADVANTAGE AF clinical trial achieves safety and effectiveness endpoints for treatment of drug-resistant, symptomatic, persistent atrial fibrillation with the FARAPULSE™ Pulsed Field Ablation System Sub-analysis from OPTION clinical trial highlights consistent safety and…
Boston Scientific Announces Agreement to Acquire Bolt Medical, Inc.
Acquisition to expand cardiovascular portfolio with complementary and differentiated calcium modification platform, furthering company’s strategy to address coronary and peripheral disease MARLBOROUGH, Mass., Jan. 8, 2025 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today…
Boston Scientific WATCHMAN FLX™ Left Atrial Appendage Closure Device Demonstrates Superior Bleeding Risk Reduction to Oral Anticoagulation Following a Cardiac Ablation in the OPTION Clinical Trial
Data also demonstrates that the stroke risk reduction device is as effective as oral anticoagulants for patients with atrial fibrillation following a cardiac ablation MARLBOROUGH, Mass. and CHICAGO, Nov. 16, 2024 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced…
Boston Scientific Announces Agreement to Acquire Cortex, Inc.
Acquisition to complement electrophysiology portfolio with solution to advance the treatment of complex atrial fibrillation MARLBOROUGH, Mass., Nov. 4, 2024 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced it has entered into a definitive agreement to acquire…
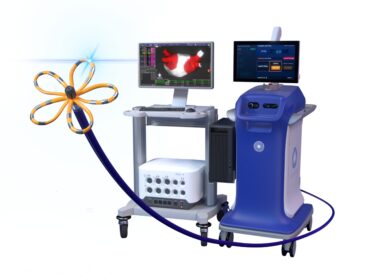
Boston Scientific Launches Next Generation of Cardiac Mapping for the FARAPULSE™ Pulsed Field Ablation System
New FARAWAVE™ NAV Ablation Catheter and FARAVIEW™ Software combine with OPAL HDx™ Mapping System to provide visualization during pulsed field ablation MARLBOROUGH, Mass., Oct. 18, 2024 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced it has received U.S. Food and…
Boston Scientific Closes Acquisition of Silk Road Medical, Inc.
MARLBOROUGH, Mass., Sept. 17, 2024 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced the close of its acquisition of Silk Road Medical, Inc. (Nasdaq: SILK), a medical device company that pioneered a new approach for stroke prevention and the treatment of carotid…