MARLBOROUGH, Mass., July 27, 2023 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) generated net sales of $3.599 billion during the second quarter of 2023, growing 11.0 percent on a reported basis, 12.0 percent on an operational1 basis and 11.6 percent on an organic2 basis, all compared to the prior year period. The company reported GAAP net income […]
Tag: Boston Scientific
Boston Scientific Elects Dr. Jessica L. Mega and Susan E. Morano to Board of Directors
MARLBOROUGH, Mass., June 27, 2023 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) announced today the election of Dr. Jessica L. Mega and Susan E. Morano to its board of directors, each effective immediately. Dr. Mega is the co-founder of Verily Life Sciences LLC, a subsidiary of Alphabet Inc. focused on life sciences and healthcare, and was the […]
Boston Scientific Announces Conversion Date of Series A Mandatory Convertible Preferred Stock
MARLBOROUGH, Mass., May 30, 2023 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) announced today that its Series A 5.50% Mandatory Convertible Preferred Stock (the preferred stock) will automatically convert into shares of the company’s common stock on June 1, 2023. As previously disclosed, holders of record at the close of business on May 15, 2023 will […]
Data at Heart Rhythm 2023 Highlight Key Boston Scientific Therapies
Additional real-world outcomes further demonstrate safety, efficacy and procedural reproducibility of the FARAPULSE™ Pulsed Field Ablation System* Results from global trial of the POLARx™ Cryoablation System* meet safety and effectiveness endpoints MARLBOROUGH, Mass., May 20, 2023 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced data supporting use of the company’s key electrophysiology […]
Boston Scientific Announces Results for First Quarter 2023
MARLBOROUGH, Mass., April 26, 2023 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) generated net sales of $3.389 billion during the first quarter of 2023, growing 12.0 percent on a reported basis, 14.9 percent on an operational1 basis and 14.0 percent on an organic2 basis, all compared to the prior year period. The company reported GAAP net income […]
BOSTON SCIENTIFIC ANNOUNCES RESULTS FOR FOURTH QUARTER AND FULL YEAR 2022
MARLBOROUGH, Mass., Feb. 1, 2023 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) generated net sales of $3.242 billion during the fourth quarter of 2022, growing 3.7 percent on a reported basis, 8.7 percent on an operational1 basis and 7.1 percent on an organic2 basis, all compared to the prior year period. Included within organic results is a […]
Boston Scientific Announces Strategic Investment to Acquire Majority Stake of Acotec Scientific Holdings Limited
MARLBOROUGH, Mass., Dec. 11, 2022 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) and Acotec Scientific Holdings Limited (“Acotec” and 6669.HK) announced today that Boston Scientific will make a partial offer to acquire a majority stake, up to a maximum of 65%, of shares of Acotec, a Chinese medical technology company that offers solutions […]
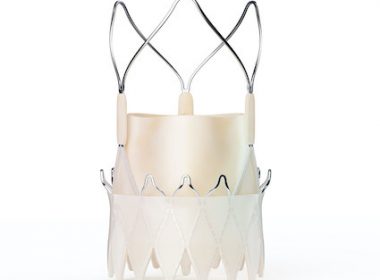
Late-Breaking Post-Market Study Data Reinforce Clinical Procedural Success and Safety of the ACURATE neo2™ Aortic Valve System
MARLBOROUGH, Mass., Nov. 27, 2022 /PRNewswire/ — Boston Scientific Corporation (NYSE:BSX) has announced the first results from the ACURATE neo2 Post Market Clinical Follow-up (PMCF) study evaluating the performance of the ACURATE neo2TM Aortic Valve System. The findings, which included a high procedural success rate of 98.4% and low rates of mortality and paravalvular […]
Boston Scientific Announces Results for Third Quarter 2022
MARLBOROUGH, Mass., Oct. 26, 2022 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) generated net sales of $3.170 billion during the third quarter of 2022, growing 8.1 percent on a reported basis, 13.7 percent on an operational1 basis and 11.5 percent on an organic2 basis, all compared to the prior year period. The company reported GAAP net income […]
Late-Breaking Data from PROTECTED TAVR Trial Demonstrate Reduced Risk of Disabling Stroke, Low Complication Rate with SENTINEL™ Cerebral Protection System
Results from largest randomized TAVR trial to date presented at TCT 2022 MARLBOROUGH, Mass., Sept. 17, 2022 /PRNewswire/ — Boston Scientific Corporation ( NYSE:BSX) has announced results from the PROTECTED TAVR clinical trial evaluating the SENTINEL™ Cerebral Protection System, which is designed to capture and remove embolic debris stemming from transcatheter aortic valve […]