Agreement expands company’s access to pulsed field ablation technology MARLBOROUGH, Mass., Sept. 21, 2020 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced it has signed an investment agreement with an exclusive option to acquire Farapulse, Inc., a privately-held company developing a pulsed field ablation (PFA) system for the treatment of atrial fibrillation […]
Tag: Boston Scientific
CMS Grants Additional Reimbursement For The Eluvia™ Drug-Eluting Vascular Stent System
MARLBOROUGH, Mass., Sept. 3, 2020 /PRNewswire/ — Boston Scientific (NYSE: BSX) announced that the U.S. Centers for Medicare and Medicaid Services (CMS) granted a New Technology Add-on Payment (NTAP) for the Eluvia™ Drug-Eluting Vascular Stent System as part of the 2021 Inpatient Prospective Payment System (IPPS). The NTAP designation, awarded to new medical […]
BioTelemetry, Inc. Announces Sales Agent Agreement with Boston Scientific Corporation
Company expands portfolio of solutions with groundbreaking insertable cardiac monitor system MALVERN, Pa., July 30, 2020 (GLOBE NEWSWIRE) — BioTelemetry, Inc. (NASDAQ:BEAT), the leading remote medical technology company focused on the delivery of health information to improve quality of life and reduce cost of care, today announced a sales agent […]
Boston Scientific Announces Results For Second Quarter 2020
MARLBOROUGH, Mass., July 29, 2020 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) generated sales of $2.003 billion during the second quarter of 2020. This represents a decline of (23.9) percent on a reported basis, (23.1) percent on an operational1 basis and (28.7) percent on an organic2 basis, all compared to the prior year period. The company reported a […]
Boston Scientific Receives FDA Approval for Next-Generation WATCHMAN FLX™ Left Atrial Appendage Closure Device
New stroke risk reduction technology designed to advance procedural performance and safety, treat wider range of patients with non-valvular atrial fibrillation MARLBOROUGH, Mass., July 21, 2020 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) announced it has received U.S. Food and Drug Administration (FDA) approval for the WATCHMAN FLX™ Left Atrial Appendage Closure (LAAC) Device. Experience […]
Boston Scientific Receives FDA 510(k) Clearance for the LUX-Dx™ Insertable Cardiac Monitor System
First ICM device with remote programming paired with dual-stage arrhythmia detection algorithm MARLBOROUGH, Mass., June 29, 2020 /PRNewswire/ — Boston Scientific (NYSE: BSX) has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the LUX-Dx™ Insertable Cardiac Monitor (ICM) System, a new, long-term diagnostic device implanted in patients to detect arrhythmias associated with […]
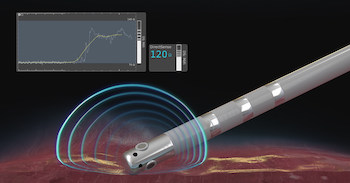
Boston Scientific Launches DIRECTSENSE™ Technology
Tool provides electrophysiologists with first-ever local measurement and visualization of tissue response to radiofrequency ablation MARLBOROUGH, Mass., June 1, 2020 /PRNewswire/ — Boston Scientific (NYSE: BSX) announced the U.S. launch of the DIRECTSENSE™ Technology, a tool for monitoring the effect of radiofrequency (RF) energy delivery during cardiac ablation procedures. Available on the RHYTHMIA HDx™ […]
PINNACLE FLX Study of the WATCHMAN FLX™ Left Atrial Appendage Closure Device Presented as Late-Breaking Clinical Trial at HRS 2020 SCIENCE
Trial meets primary safety and efficacy endpoints with low complication rates, 100% effective closure with next-generation stroke risk reduction technology for patients with non-valvular atrial fibrillation MARLBOROUGH, Mass., May 8, 2020 /PRNewswire/ — Today, Boston Scientific (NYSE: BSX) announced positive 12-month results from the PINNACLE FLX clinical trial assessing the safety and efficacy […]
Boston Scientific Announces Results For First Quarter 2020
MARLBOROUGH, Mass., April 29, 2020 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) generated sales of $2.543 billion during the first quarter of 2020. This represents growth of 2.0 percent on a reported basis, 3.2 percent on an operational1 basis and a decline of 2.9 percent on an organic2 basis, all compared to the prior year period. The […]
BioCardia Announces Litigation Financing in the Case Captioned Boston Scientific Corp., et al., v. BioCardia Inc.
SAN CARLOS, Calif., April 14, 2020 (GLOBE NEWSWIRE) — BioCardia®, Inc. (Nasdaq: BCDA), a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today reported it has entered into an agreement for litigation financing which has been filed today with the Securities and Exchange Commission on Form 8-K. BioCardia, Inc. […]