SANTA CLARA, Calif. – September 15, 2025 – Today, Johnson & Johnson announced the European launch of its Shockwave Javelin Peripheral IVL Catheter, a novel intravascular lithotripsy (IVL) platform designed to modify calcium in extremely narrowed vessels to expand treatments in patients suffering from peripheral artery disease (PAD). The first-of-its-kind Forward IVL […]
Tag: BTK
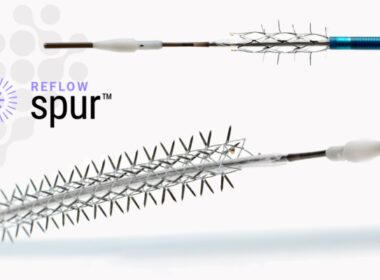
Reflow Medical Receives CE Mark for Bare Temporary Spur Stent System for Treating de novo or Restenotic Below-the-Knee (BTK) Lesions
San Clemente, CA—Jan. 15, 2024 – Reflow Medical, Inc., a developer of innovative medical devices focused on cardiovascular disease, announces it has received CE (Conformité Européenne) Mark certification in the European Union for the Bare Temporary Spur Stent System. The device is intended to treat de novo or restenotic lesions […]
TOBA II BTK Trial Enrollment Ahead of Schedule for the Tack Endovascular System® in Below the Knee Disease
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced the Tack Optimized Balloon Angioplasty II Below the Knee (TOBA II BTK) clinical trial surpassed the 100 patient milestone, advancing enrollment well ahead of schedule. The TOBA II BTK is a prospective, […]
BD Announces Completion of Enrollment in the Lutonix 014 Drug Coated Balloon Below-the-Knee Trial
FRANKLIN LAKES, N.J., Jan. 18, 2018 /PRNewswire/ — Becton, Dickinson and Company (NYSE: BDX), a leading global medical technology company, today announced the completion of enrollment in the Lutonix below-the-knee (BTK) trial and plans to submit a pre-market approval application in late 2018. The Lutonix BTK trial is a prospective, multicenter, randomized, […]
Nitiloop Announces FDA 510(k) Clearance for its Nova Cross Extreme and Nova Cross BTK Micro catheter for Support of Guidewire Access to Discrete Regions of the Coronary and Peripheral Vasculature
NETANYA, Israel, October 26, 2017 /PRNewswire/ — Nitiloop, a medical device company dedicated to the development of Cardiovascular and peripheral microcatheters for complex lesions, received FDA clearance for its new Nova Cross™ Extreme and Nova Cross™ BTK. These dedicated microcatheters are joining the Nova Cross™ product family combining innovative low profile microcatheter technology with uniquely designed Nitinol scaffold providing […]
Shockwave Medical Shows Off Disrupt BTK Lithoplasty System Study Results
FREMONT, Calif.–(BUSINESS WIRE)–Shockwave Medical, a pioneer in the treatment of calcified cardiovascular disease, today reported positive results from the DISRUPT BTK Study, which were presented at the annual Cardiovascular and Interventional Radiological Society of Europe (CIRSE) congress in Copenhagen, Denmark. DISRUPT BTK, a prospective single arm study, evaluated the use […]
Angiolite BTK, below the knee sirolimus-eluting stent, changed CE Mark Approval
(BTK), improving blood flow in severe claudication and critical limb ischaemia. Angiolite BTK design has been specifically elaborated for drug eluting stent and benefits from iVascular proprietary coating nanotechnology that yields a multilayer thin coating with optimal kinetics. “Having the right bail-out options, when performing BTK angioplasty, is extremely important in […]
Intact Vascular Announces Enrollment Of First European Patient In Tack Optimized Balloon Angioplasty II Below The Knee (TOBA II BTK) Clinical Trial With The Tack Endovascular System
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced that its Tack Optimized Balloon Angioplasty II Below the Knee (TOBA II BTK) clinical trial has commenced enrollment in Europe, with the first patient treated by Professor Dr. Marianne Brodmann and Dr. Peter Reif at Medical University Graz, Austria. […]
Intact Vascular Release: FDA Approves 6-Month Primary Endpoint For The Tack Endovascular System In Below The Knee Disease
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced the U.S. Food and Drug Administration (FDA) approved an Investigational Device Exemption (IDE) supplemental application to modify the primary endpoint in the Tack Optimized Balloon Angioplasty II Below the Knee (TOBA II BTK) clinical trial from 12 months to […]