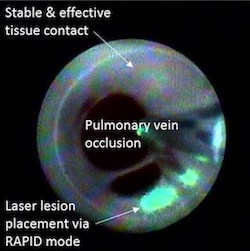
– China Grand Pharma will seek regulatory approvals and commercialize CardioFocus’ innovative catheter ablation technology for pulmonary vein isolation – Pulmonary vein isolation is the gold standard treatment for atrial fibrillation MARLBOROUGH, Mass., June 2, 2021 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatments for atrial fibrillation (AFib), […]
Tag: CardioFocus
CardioFocus Announces The Publication Of HeartLight X3 Pivotal Study Data
HeartLight® X3 System Has Significantly Shorter Procedure Times for Atrial Fibrillation Ablation MARLBOROUGH, Mass., Feb. 25, 2021 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), announced today that results from its HeartLight® X3 Pivotal Study were published in the premier journal Circulation: Arrhythmia and Electrophysiology (CircEP), which features […]
CardioFocus Announces Expanded Partnership With Japan Lifeline
HeartLight® X3 Japan Launch Anticipated in Mid-2021 MARLBOROUGH, Mass., Feb. 2, 2021 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatments for atrial fibrillation (AFib), announced today that it has expanded its existing partnership agreement with Japan Lifeline Co., Ltd. (JLL). The agreement extends the timeframe of the existing distribution […]
CardioFocus® Announces Expanded Distribution Partnership With MicroPort™ CRM To Include Spain And Portugal
MARLBOROUGH, Mass., Jan. 11, 2021 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), announced today that it has expanded its existing French distribution partnership agreement with MicroPort CRM in France to include both Spain and Portugal. “We are pleased to be represented by MicroPort CRM in France and have been very impressed by […]
CardioFocus Announces 10,000 Patients Treated Worldwide
HeartLight Procedure Advances the Treatment of Atrial Fibrillation MARLBOROUGH, Mass., Dec. 15, 2020 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), announced today that more than 10,000 patients worldwide have been treated with the HeartLight® Endoscopic Ablation System. The HeartLight System is a revolutionary catheter […]
CardioFocus® Announces Initial Commercial Procedures Following US FDA Approval of HeartLight® X3 System for the Treatment of Atrial Fibrillation
MARLBOROUGH, Mass., June 16, 2020 /PRNewswire/ — CardioFocus, Inc. today announced that the first U.S. patients have been treated commercially with the recently-approved HeartLight® X3 Endoscopic Ablation System. The revolutionary cardiac ablation technology is designed to treat drug refractory, symptomatic paroxysmal atrial fibrillation (AFib), the most common heart rhythm disorder. Henry D. Huang, M.D., FACC, […]
CardioFocus Announces US FDA Approval of HeartLight® X3 System for the Treatment of Atrial Fibrillation
MARLBOROUGH, Mass., May 12, 2020 /PRNewswire/ — CardioFocus, Inc. today announced that the U.S. Food and Drug Administration (FDA) has approved the next-generation HeartLight® X3 Endoscopic Ablation System for the treatment of drug refractory recurrent symptomatic paroxysmal atrial fibrillation (PAF). Approval of the HeartLight X3 System came as a result of a comprehensive submission, including outcomes […]
CardioFocus Announces U.S. FDA PMA Supplement Submission For The Breakthrough HeartLight X3 Endoscopic Ablation System
MARLBOROUGH, Mass., Feb. 4, 2020 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), today announced that the company has submitted a pre-market approval (PMA) supplement to the U.S. Food and Drug Administration (FDA) for the HeartLight X3 Endoscopic Ablation System for the treatment of AFib. This […]
CardioFocus® Names Burke T. Barrett As Chief Executive Officer
MARLBOROUGH, Mass., Jan. 22, 2020 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), announced today that the company’s Board of Directors has promoted Burke T. Barrett to the position of Chief Executive Officer (CEO), effective January 19, 2020. Mr. Barrett previously served as CardioFocus’ President and […]
CardioFocus® Treats First Patients With HeartLight® Endoscopic Ablation System In France
MARLBOROUGH, Mass., Oct. 29, 2019 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), announced today that the first patients in France have been treated in the University Public Hospital of Nancy with the HeartLight Endoscopic Ablation System as part of an exclusive distribution partnership with MicroPort® CRM France. To […]