UK’s BTG to buy Roxwood Medical for up to $80 million Reuters Staff Reporting by Radhika Rukmangadhan in Bengaluru; editing by Jason Neely Our Standards:The Thomson Reuters Trust Principles. (Reuters) – British drugs company BTG Plc (BTG.L) said on Thursday it will buy U.S.-based cardiovascular catheter maker Roxwood Medical for […]
Tag: cardiovascular
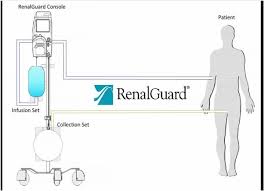
Meta-Analysis Shows RenalGuard(R) Offers Dramatic Benefit for Preventing Contrast-Induced Acute Kidney Injury in Patients Undergoing Cardiovascular Interventions
MILFORD, MA–(Marketwired – September 13, 2017) – A new meta-analysis suggests that the use of the RenalGuard System® reduces the rate of contrast-induced acute kidney injury (CI-AKI) compared to standard intravascular volume expansion in moderate-to-high-risk patients with chronic kidney disease who are undergoing cardiac interventional procedures. The study, published in the Journal […]
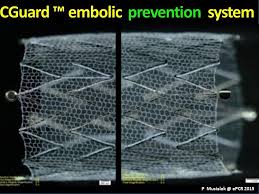
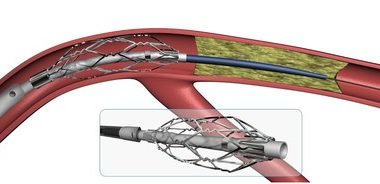
InspireMD Shares CGuard EPS Video Testimonials From European Key Opinion Leaders
TEL AVIV, ISRAEL–(Marketwired – August 22, 2017) – InspireMD, Inc. (NYSE American: NSPR) (NYSE MKT: NSPR) (NYSE American: NSPR.WS) (NYSE MKT: NSPR.WS) (“InspireMD” or the “Company”), a leader in embolic prevention systems (EPS) / thrombus management technologies and neurovascular devices, today announced it has posted video testimonials about its CGuard™ Embolic Prevention System from […]
Roxwood Medical Announces Agreement With Abbott (ABT) For Distribution Of Products In U.S.
REDWOOD CITY, Calif., July 25, 2017 /PRNewswire/ — Roxwood Medical Inc., a leading provider of advanced cardiovascular specialty catheters, today announced it has entered into an exclusive agreement with Abbott for distribution of Roxwood products in the United States. “This partnership with Abbott’s vascular business is a major milestone for Roxwood that allows far […]
New Meta-Analysis Presented at American College of Cardiology Strongly Supports RenalGuard(R) Use in Cardiovascular Interventional Procedures
MILFORD, MA — (Marketwired) — 03/22/17 — A new meta-analysis presented this week at the American College of Cardiology further supports the ability of RenalGuard Therapy® to significantly reduce the incidence of acute kidney injury associated with cardiovascular interventional procedures compared to other methods of urine volume expansion. Moreover, findings […]
Tryton Medical Announces First U.S. Commercial Case with Tryton Side Branch Stent
DURHAM, N.C.–(BUSINESS WIRE)– Tryton Medical, Inc., the leading developer of stents designed to treat coronary bifurcation lesions, today announced that the first U.S. commercial case using the Tryton Side Branch Stent to treat a coronary bifurcation lesion involving a large side branch (appropriate for a ≥2.5mm stent) was completed at […]
Toshiba Medical Systems’ New Premium Cardiovascular Ultrasound Wins FDA Approval
Toshiba Medical’s New Premium Cardiovascular Ultrasound Receives FDA Clearance The Aplio i900 Delivers Fast, High Quality Images for Better Diagnostic Confidence TUSTIN, Calif.–(BUSINESS WIRE)–Cardiologists can now access the advanced ultrasound imaging technology needed for fast and confident diagnoses with Toshiba Medical’s Aplio™ i900. The newly FDA-cleared system is the latest […]
Teleflex (TFX) Announces 510(k) Clearance and Global Launch of Twin-Pass® Torque Dual Access Catheter
Dual access catheter enables a 0.014″ guidewire to remain in place while delivering contrast, medication, or a second 0.014″ guidewire – new Torque version builds on Vascular Solutions’ long-standing Twin-Pass Catheter platform with enhanced torque response and precise angle alignment into side branch vessels WAYNE, Pa.–(BUSINESS WIRE)–Teleflex Incorporated (NYSE: TFX), […]
Teleflex (TFX) Announces 510(k) Clearance and U.S. Launch of Spectre™ Guidewire
View source version on businesswire.com: http://www.businesswire.com/news/home/20170314005152/en/ Teleflex Incorporated (TFX), a leading global provider of medical technologies for critical care and surgery, has announced 510(k) clearance by the Food and Drug Administration and U.S. commercial launch of the Spectre Guidewire. The Spectre Guidewire is engineered with a smooth stainless steel-to-nitinol dual-core […]