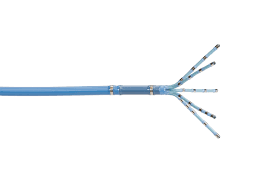
The OCTARAY™ Mapping Catheter with TRUEref™ Technology provides physicians with the precise information needed for catheter ablation procedures that treat cardiac arrhythmias1,2† IRVINE, Calif., Sept. 6, 2022 /PRNewswire/ — Biosense Webster, Inc., part of Johnson & Johnson MedTech‡, today announced the release of the OCTARAY™ Mapping Catheter with TRUEref™ Technology powered by the […]
Tag: Carto 3
With Innovative Health’s landmark clearance to reprocess the leading electrophysiology mapping catheter from Biosense Webster, single-use device reprocessing has entered a new era.
SCOTTSDALE, Ariz., June 28, 2019 /PRNewswire-PRWeb/ — Innovative Health, the leading single-use cardiology medical device reprocessing company, today announced it has received FDA clearance for reprocessing the market-leading PENTARAY® Nav eco High-Density mapping catheter (hereinafter PentaRay), a development that represents both a technological and healthcare cost reduction milestone. The PentaRay is a key […]
Biosense Webster Launches CARTO® 3 System CARTO VISITAG™ Module with Ablation Index, designed to help Electrophysiologists standardize and simplify the treatment of Atrial Fibrillation
DIAMOND BAR, California, March 22, 2017 /PRNewswire/ — Biosense Webster, a Division of Johnson & Johnson Medical NV/SA, a worldwide leader in the diagnosis and treatment of cardiac arrhythmias, announced today the launch of the CARTO VISITAG™ Module with Ablation Index, a new technology providing visual indication based on the […]