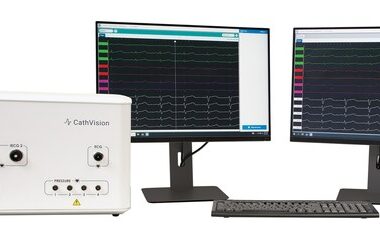
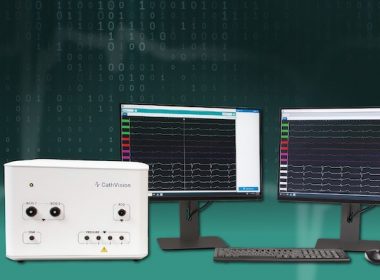
COPENHAGEN, Denmark, Aug. 15, 2023 /PRNewswire/ — CathVision, a medical technology company developing innovative electrophysiology solutions designed to enhance clinical decision making in the EP lab, today announced the FDA clearance and commercial availability of the PVI AnalyzerTM and Signal ComplexityTM algorithms. These algorithms are part of the CARDIALYTICSTM suite of artificial intelligence-powered analytics integrated into […]
Tag: CathVision
CathVision Secures $9 Million in Funding to Accelerate Adoption of ECGenius System & CARDIALYTICS AI-Suite
COPENHAGEN, Denmark, July 18, 2023 /PRNewswire/ — CathVision, a medical technology company developing innovative electrophysiology solutions designed to support clinical decision making in the EP lab, today announced its most recent financing round of $9 million from investors. CathVision secures $9 Million in funding to advance commercial operations driving adoption of the ECGenius™ System The […]
Progressing Clinical Evidence: CathVision Announces First Patient Enrolled In Persistent AF Study at OLV Hospital Aalst
Series of Clinical Studies Highlight Advantages of ECGenius System and CARDIALYTICS Suite of Algorithms COPENHAGEN, Denmark, April 17, 2023 /PRNewswire/ — CathVision, a medical technology company developing innovative electrophysiology solutions to enhance clinical decision-making in the EP lab, today announced initial patient enrollment of a follow-on clinical study to demonstrate the value […]
CathVision Announces Key Milestones in Advance of ECGenius Commercial Launch
ECGenius System & CARDIALYTICS Suite Recognized as Critical to the Evolution of Electrophysiology COPENHAGEN, Denmark, March 28, 2023 /PRNewswire/ — CathVision, a medical technology company developing innovative electrophysiology solutions designed to enhance clinical decision-making in the EP lab, is proud to announce the achievement of significant milestones demonstrating both the clinical and commercial […]
CathVision Announces Initiation of Clinical Study Evaluating Signal Complexity Algorithm
Study Advances Efforts to Launch CARDIALYTICS Suite of Analytic Tools COPENHAGEN, Denmark, Nov. 2, 2022 /PRNewswire/ — CathVision, a medical technology company developing innovative electrophysiology solutions to enhance clinical decision-making in the EP lab, today announced the investigation of the Signal Complexity™ algorithm designed to visualize and quantify atrial fibrillation (AF) complexity parameters in […]
CathVision Announces Close Of $7.2 Million Financing Round
Funding Will Accelerate Commercialization of the ECGenius™ System and Expand Development of Analytic Modules COPENHAGEN, Denmark, Aug. 17, 2022 /PRNewswire/ — CathVision, a medical technology company developing innovative electrophysiology solutions designed to enhance clinical decision making in the EP lab, today announced the company has secured $7.2 million in funding from existing investors. The financing […]
CathVision Announces FDA Clearance of ECGenius™ High-Fidelity, Low-Noise EP Recording Technology
ECGenius Sets a New Standard for ECG Signal Detection, Interpretation and Therapy Support COPENHAGEN, Denmark , May 4, 2022 /PRNewswire/ — CathVision, a medical technology company developing innovative electrophysiology solutions designed to enhance clinical decision making in the EP lab, today announced the FDA 510(k) clearance of the ECGenius EP Recording System. ECGenius, the […]
CathVision Announces Completion of Patient Enrollment at First U.S. Clinical Trial Evaluating High-Fidelity, Low-Noise EP Recording Technology
University of Vermont Medical Center Initiates Evaluation of the ECGenius System as CathVision Prepares for U.S. Launch COPENHAGEN, Denmark, April 26, 2022 /PRNewswire/ — CathVision, a medical technology company developing innovative electrophysiology solutions designed to guide and enhance cardiac ablation therapy through the acquisition of low-noise, high-fidelity electrograms, today announced the completion of patient […]
CathVision Announces First Patient Enrollments in PVISION Multicenter Clinical Study
Researchers to Examine Efficacy of Using AI Algorithm to Confirm Pulmonary Vein Isolation COPENHAGEN, Denmark and MINNETONKA, Minn., Oct. 26, 2021 /PRNewswire/ — CathVision, a medical technology company developing innovative electrophysiology solutions in EP recording technology, today announced the first patient enrollments in the PVISION multicenter clinical study. The newly launched trial will investigate the […]
CathVision adds US profile to its Board of Directors
COPENHAGEN, Denmark, March 16, 2021 /PRNewswire/ — CathVision ApS, a medical device company developing a novel artificial intelligence therapeutic platform for cardiac electrophysiology procedures, is announcing a change to its Board of Directors. Meghna Eichelberger, Partner and Associate Director of Medical Technologies, at The Boston Consulting Group (BCG), has joined CathVision’s Board […]