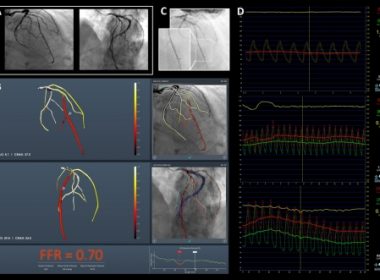
KFAR-SABA, Israel and ALISO VIEJO, Calif., June 14, 2021 /PRNewswire/ — CathWorks, a global leader of digital health innovation focused on helping patients with cardiovascular disease, today announced that Ramin Mousavi has been appointed Chief Executive Officer, effective June 21, 2021. Mr. Mousavi has also been appointed to the company’s Board of Directors. Mr. Mousavi succeeds Jim Corbett, who […]
Tag: CathWorks
CathWorks FFRangio™ System Receives Regulatory Approval in Japan
KFAR-SABA, Israel and ALISO VIEJO, Calif., Dec. 11, 2019 /PRNewswire/ — CathWorks today announced the approval of The CathWorks FFRangio™ System by the Japan’s Ministry of Health, Labour and Welfare (MHLW). The CathWorks FFRangio System is a non-invasive diagnostic technology that is used at the time of a routine angiography. The CathWorks FFRangio System transforms routine angiogram images […]
CathWorks Appoints Chief Financial Officer
KFAR-SABA, ISRAEL & ALISO VIEJO, Calif.–(BUSINESS WIRE)–CathWorks announced that Mike Feher has joined CathWorks as Vice President of Finance and Chief Financial Officer. His appointment coincides with US commercialization of the CathWorks FFRangio™ System. Mr. Feher will initially focus on the development of financial and operational capabilities, as well as creating […]
CathWorks Adds Vice President of Global Marketing to Leadership Team
KFAR-SABA, Israel & ALISO VIEJO, Calif.–(BUSINESS WIRE)–CathWorks announced that Ramin Mousavi has joined CathWorks as Vice President of Global Marketing & Strategy. Mr. Mousavi, an experienced cardiovascular marketer, will take responsibility for the CathWorks FFRangio™ System U.S. clinical and commercial launch and direct future expansion into other global markets. Mr. Mousavi […]
CathWorks Announces Completion of $30 Million Financing
KFAR-SABA, Israel & ALISO VIEJO, Calif.–(BUSINESS WIRE)–CathWorks announced the completion of a $30 million Series C financing round led by Deerfield Management. Jim Corbett, CathWorks CEO, stated, “We are delighted to have such a notable investor leading this round and for the continued full participation of our existing syndicate of […]
CathWorks Names Paul Kapsner as Vice-President of Sales
KFAR-SABA, Israel & ALISO VIEJO, Calif.–(BUSINESS WIRE)–CathWorks announced that Paul Kapsner has been named as Global Vice President of Sales. His appointment coincides with recent United States FDA clearance of the company’s CathWorks FFRangio™ System. His initial focus will be on accelerating the clinical and commercial organization in the United States. […]
CathWorks FFRangio™ System Receives U.S. FDA Clearance
KFAR-SABA, Israel & ALISO VIEJO, Calif.–(BUSINESS WIRE)–CathWorks announced today that its FFRangio™ System received United States Food & Drug Administration (FDA) 510(k) clearance. The FFRangio system demonstrated accuracy versus the invasive FFR wire in a blinded comparative study, FAST-FFR. The results of the FAST-FFR pivotal study were used to establish substantial […]
CathWorks FFRangio™ Trial Meets Primary Endpoint and Exceeds Performance Goals
KFAR-SABA, Israel–(BUSINESS WIRE)–The journal Circulation published results from the CathWorks FFRangio™ FAST-FFR clinical trial in an article titled Accuracy of Fractional Flow Reserve Derived From Coronary Angiography. The publication and trial results were announced today at the 2018 TCT (Transcatheter Cardiovascular Therapeutics) annual meeting in San Diego, California. The FAST-FFR trial demonstrated that the […]
FAST-FFR Pivotal Trial Added to TCT 2018 Late-Breaking Clinical Trial Program
KFAR-SABA, Israel–(BUSINESS WIRE)–CathWorks announced that the FAST-FFR pivotal trial has been added to the late-breaking clinical science program during the 2018 TCT (Transcatheter Cardiovascular Therapeutics) annual meeting in San Diego. FAST-FFR trial data will be presented in the Main Arena during the late-breaking science session that begins at 12 noon […]
CathWorks FFRangio™ System Files FDA 510(k) Submission
KFAR-SABA, Israel–(BUSINESS WIRE)–CathWorks announced that it has submitted its CathWorks FFRangio™ System to the United States Food & Drug Administration (FDA) for review and 510(k) market clearance. The CathWorks FFRangio System is the non-invasive FFR platform that quickly and precisely delivers objective multi-vessel physiologic measurements to cost-effectively optimize and confirm intraprocedural PCI […]