The U.S. trial for CE-marked CBSO is designed to enroll up to 250 patients in a staged study approach BAAR, Switzerland, July 1, 2020 /PRNewswire/ — CARAG AG, a privately-held Swiss medical device development company, today announced receiving U.S. Food and Drug Administration (FDA) Investigational Device Exemption (IDE) approval for its Carag Bioresorbable […]
Tag: CBSO
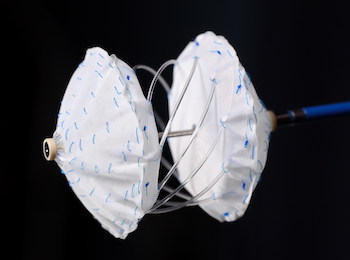
Carag First-ever Septal Occluder With Bioresorbable, Metal-free Framework Available to Patients Through New European Registry
First 3-year-old patient successfully implanted and discharged from hospital in Berlin BAAR, Switzerland and BERLIN, March 10, 2020 /PRNewswire/ — CARAG AG, a Swiss privately held medical device development company, announced today the successful implantation of the first-ever transcatheter septal occluder with bioresorbable, metal-free framework, as part of a new international post-market registry. The […]
Innovative implant for occlusion of heart defects
CARAG Completes CE Marking for its Breakthrough Carag Bioresorbable Septal Occluder (CBSO) Innovative bioresorbable implant of Carag removes risks associated with conventional metal frameworks and expands treatment options for patients with heart defects. CARAG AG, a privately held medical device development company, today announced that it has completed CE (Conformité […]