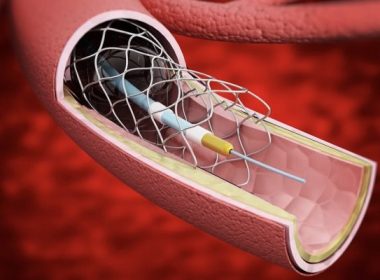
Paris, France, January 26, 2026 – HighLife SAS, a leading MedTech company focused on transcatheter solutions for structural heart disease, today announced that it has received CE Mark approval for the HighLife Transcatheter Mitral Valve Replacement (TMVR) System for the treatment of adult patients suffering from symptomatic moderate-severe or severe mitral valve regurgitation (MR), […]
Tag: CE Mark
Abbott receives CE Mark for the TactiFlex™ Duo Ablation Catheter to treat patients with abnormal heart rhythms
Abbott’s TactiFlex™ Duo Ablation Catheter, Sensor Enabled™, is designed with dual-energy to treat atrial fibrillation patients with the most challenging cases It can deliver both radiofrequency energy and pulsed field ablation (PFA) energy during procedures to target and treat an…
Microbot Medical Engaging with a Leading Notified Body to Advance CE Mark for Sales in Europe
HINGHAM, Mass., July 25, 2023 (GLOBE NEWSWIRE) — Microbot Medical Inc. (Nasdaq: MBOT), the developer of the LIBERTY® Robotic Surgical System, the first single-use endovascular robotic system, today announced the first steps towards its planned European market clearance, by engaging with a leading Notified Body. The Notified Body will audit the […]
Caption Health Receives CE Mark for Caption AI™ technology platform
Further milestone for company as it fulfills its mission to detect cardiac disease earlier in patients BRISBANE, Calif., July 19, 2022 /PRNewswire/ — Caption Health, the leader in using AI and services to improve heart ultrasound access, today announced that it has received a CE Mark for its Caption AI™ technology platform. This […]
REVA ANNOUNCES CE MARK AND FIRST IMPLANT OF THE FANTOM ENCORE BIORESORBABLE SCAFFOLD
2.5 millimeter Diameter Size with Market-Leading 95 micron Strut Profile Secures Early Approval San Diego, California (Monday, February 26, 2018 – PST) – REVA Medical, Inc. (ASX: RVA) (“REVA” or the “Company”), a leader in bioresorbable polymer technologies for vascular applications, announces CE Mark of the 2.5 millimeter diameter size […]
Abbott Introduces Next Generation of Most Widely Used Heart Stent for People With Coronary Artery Disease in Europe
ABBOTT PARK, Ill., Oct. 30, 2017 /PRNewswire/ — Abbott today announced it received CE Mark for XIENCE Sierra, the newest generation of the company’s gold-standard XIENCE everolimus-eluting coronary stent system. CE Mark allows sale of the device in the European Union and other countries that recognize CE Mark. Advances in this generation […]
Innovative implant for occlusion of heart defects
CARAG Completes CE Marking for its Breakthrough Carag Bioresorbable Septal Occluder (CBSO) Innovative bioresorbable implant of Carag removes risks associated with conventional metal frameworks and expands treatment options for patients with heart defects. CARAG AG, a privately held medical device development company, today announced that it has completed CE (Conformité […]
Gecko Biomedical receives CE Mark Approval for SETALUM™ Sealant
Approval of CE Mark paves the way for application expansion and the exploration of new therapeutic areas for ground-breaking surgical solutions. Paris, France, September 11, 2017 – Gecko Biomedical (“Gecko”), a medical device company developing innovative polymers to support tissue reconstruction, announced today that it has received CE Mark approval […]
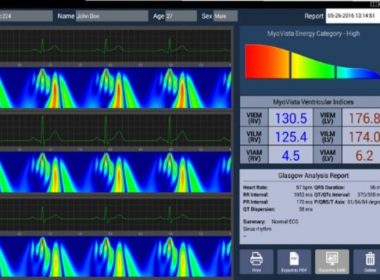
HeartSciences Announces CE Mark and European Launch of MyoVista® High Sensitivity ECG Device
WESTLAKE, Texas, Aug. 17, 2017 /PRNewswire/ — HeartSciences today announced the European launch of MyoVista® high sensitivity electrocardiograph (hsECG™) Testing Device, developed in response to the global unmet need for effective, low-cost, front-line screening of cardiac disease in both symptomatic and asymptomatic patients. MyoVista measures the heart’s energy during each […]
Cordis, a Cardinal Health Company, introduces innovative products at EUROPCR 2017 to rapidly expand interventional cardiology offering
PCRonline/News/Industry Press Releases – 15 May, 2017 DUBLIN, Ohio. Cordis, Cardinal Health‘s interventional vascular business, announced today the commercial launch of three new products that further enhance its interventional cardiology product portfolio in Europe, the Middle East, and Africa (EMEA). The addition of the Cordis RAILWAY Sheathless Access System as well […]