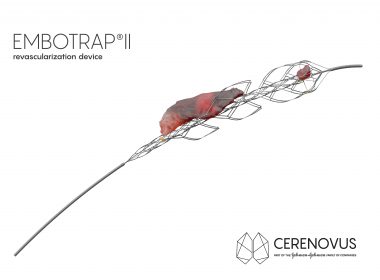
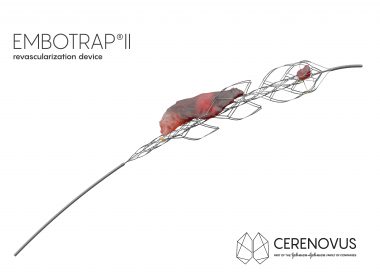
EXCELLENT study demonstrates real-world benefits of CERENOVUS’ EMBOTRAP™ Revascularization Devices on clinical outcomes and first pass reperfusion IRVINE, Calif., Nov. 18, 2022 /PRNewswire/ — CERENOVUS, Inc., part of Johnson & Johnson MedTech*, today announced positive primary outcomes from the real-world EXCELLENT Registry focused on stroke-inducing blood clot removal by mechanical thrombectomy at the Society […]
Tag: Cerenovus
CERENOVUS LAUNCHES LARGEST GLOBAL REGISTRY TO STUDY STROKE-INDUCING BLOOD CLOTS REMOVED BY THROMBECTOMY
Company Also Receives European CE Mark Approval for Novel Stroke Technology Designed for Difficult to Extract Blood Clots IRVINE, Calif., Dec. 10, 2018 — Johnson & Johnson Medical Devices Companies* today announced that its CERENOVUS business has launched the single largest global registry, the EXCELLENT Registry, to collect and analyze […]
CERENOVUS Receives European Regulatory Approval For BRAVO Flow Diverter to Treat Intracranial Aneurysms.
BRUSSELS–(BUSINESS WIRE)–CERENOVUS, part of the Johnson & Johnson Medical Devices Companies, announced today it has received European CE mark approval for its BRAVO Flow Diverter. The device is approved for use in the treatment of patients suffering from intracranial aneurysms. The device will divert blood flow from the aneurysm and […]
First Ischemic Stroke Patients Treated With EMBOTRAP II Revascularization Device Since Commercial Availability In U.S.
SAN FRANCISCO, July 24, 2018 /PRNewswire/ — CERENOVUS, part of the Johnson & Johnson Medical Devices Companies, announced the first patients have been treated with its new EMBOTRAP II Revascularization Device since it became commercially available in the U.S. EMBOTRAP II is a next generation stent retriever used to capture and remove life-threatening blood clots […]
CERENOVUS Receives FDA Clearance For Next Generation Stent Retriever Device Used To Treat Ischemic Stroke
IRVINE, Calif., May 21, 2018 /PRNewswire/ — CERENOVUS, part of the Johnson & Johnson Medical Devices Companies, announced today it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for EMBOTRAP II Revascularization Device, a next generation stent retriever used to capture and remove life-threatening blood clots from […]
NEW STUDY SHOWS USE OF EMBOTRAP® DEVICE CLOT RETRIEVER DURING TREATMENT OF STROKE YIELDS POSITIVE RATES OF REVASCULARIZATION
ARISE II Clinical Trial Results Presented at International Stroke Conference LOS ANGELES – Jan. 25, 2018 – EMBOTRAP® Device, a device designed to remove clots from the brain following an ischemic stroke, demonstrated high success rates in restoring blood flow and patients achieved high rates of functional independence, according to […]
GALAXY G3 MINI Coil is the Smallest and Softest Finishing Coil the Company Has Ever Produced
IRVINE, CA – Jan. 24, 2018 – CERENOVUS, part of the Johnson & Johnson Medical Devices Companies, today announced the launch of the GALAXY G3 MINI Coil, its smallest and softest embolic finishing coil, for use in the endovascular treatment of cerebral aneurysms and hemorrhagic stroke. The company received 510 […]
Johnson & Johnson Medical Devices Companies Signals Commitment to Stroke Treatment With the Announcement of New Neurovascular Business
NICE, France, September 7, 2017 /PRNewswire/ — Johnson & Johnson Medical Devices Companies* today introduced CERENOVUS,** its new neurovascular business, at the European Society of Minimally Invasive Neurological Therapy (ESMINT) 9th Annual Meeting. CERENOVUS, a name derived from the Latin words for ‘new’ and ‘brain’, will focus on delivering innovative therapies for hemorrhagic […]
Pulsar and Neuravi Now Part of J&J’s New Cerenovous Neurovascular Business
Johnson & Johnson rolls Pulsar Vascular, Neuravi into new Cerenovus neurovascular biz JULY 25, 2017 BY BRAD PERRIELLO – MassDevice Johnson & Johnson (NYSE:JNJ) said yesterday that it’s medical device division is rolling the Pulsar Vascular and Neuravi acquisitions into a new neurovascular business dubbed Cerenovus. The New Brunswick, N.J.-based healthcare giant said the new brand, unveiled at […]