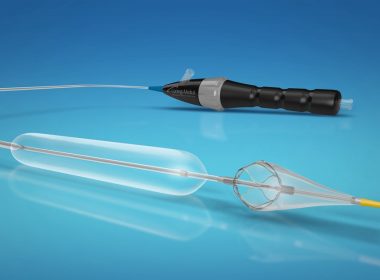
RALEIGH, N.C., Dec. 7, 2018 /PRNewswire/ — Contego Medical announced today that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its Vanguard IEP® Peripheral Balloon Angioplasty System with Integrated Embolic Protection. Contego Medical is a medical device company developing and commercializing a suite of next-generation devices that address […]
Tag: Contego
Contego Medical Receives 510(k) Clearance for the Paladin Carotid PTA Balloon System with Integrated Embolic Protection
RALEIGH, N.C., Sept. 18, 2018 /PRNewswire/ — Contego Medical announced today that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the first filter-based Integrated Embolic Protection (IEP) device, the Paladin® Carotid PTA Balloon System. Contego Medical is developing and commercializing a suite of next-generation devices that address unmet needs in […]