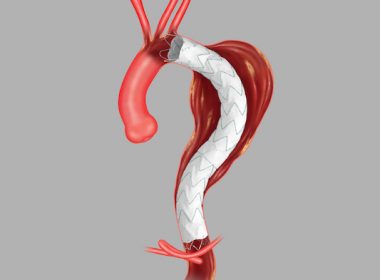
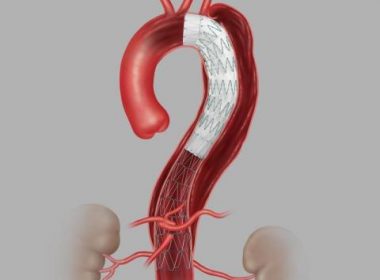
Bloomington, Ind. — Cook Medical’s Zenith® Thoraco+ Endovascular System (Thoraco+) has received Breakthrough Device Designation from the US Food and Drug Administration (FDA). This designation is granted to devices that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions. While the product is […]
Tag: Cook Medical
Economic Impact of Cook Medical’s new Indy manufacturing site estimated at $25.9 Million
Indianapolis, Ind. – Indiana University’s Public Policy Institute (PPI) estimates that Bloomington, Indiana based Cook Medical’s medical device manufacturing facility nearing completion at 38th & Sheridan in Indianapolis will have an estimated economic impact of $25.9 million annually for Marion County. The $15 million, 40,000 sq. foot facility, which plans to begin […]
Cook Medical receives FDA breakthrough designation for new drug-eluting stent
Bloomington, Ind. — Cook Medical has received Breakthrough Device designation from the US Food and Drug Administration (FDA) on a new drug-eluting stent for below the knee (BTK). This new stent is designed to treat patients suffering from chronic limb-threatening ischemia (CLTI). “CLTI is a debilitating disease of growing prevalence around […]
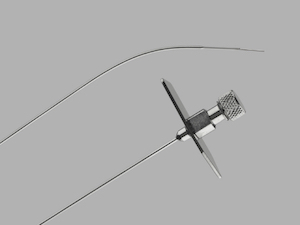
Cook Medical Issues Voluntary Recall of Transseptal Needle and Transseptal Needle With Catheter
BLOOMINGTON, Ind.–(BUSINESS WIRE)–Cook Medical issued a global, voluntary recall of the Transseptal Needle and the Transseptal Needle with Catheter. This recall includes all unexpired lots for both of these products. The needles were recalled due to complaints of rust on the products. Use of affected products could result in increased […]
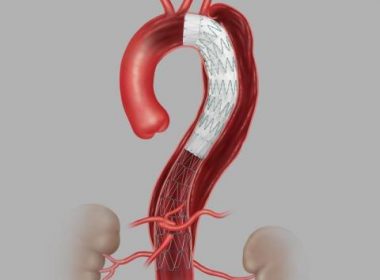
Cook Medical receives FDA Breakthrough Device Designation for Zenith® Fenestrated+ Endovascular Graft
Bloomington, Ind. — Cook Medical’s Zenith® Fenestrated+ Endovascular Graft (ZFEN+) product has received Breakthrough Device designation from the US Food and Drug Administration (FDA). This designation is granted for devices that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions. While the […]
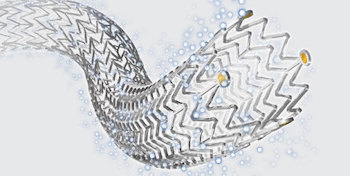
Data Show Zilver® PTX® Leads to Fewer Complications and Shorter Hospital Stays Than Traditional Bypass Surgery
BLOOMINGTON, Ind.–(BUSINESS WIRE)–At this year’s VEITHsymposium®, Dr. Marc Bosiers presented data that show that patient treatment with the Zilver® PTX® stent has several benefits when compared to traditional bypass surgery. The data, which were gathered from a randomized controlled trial, show that treatment with Zilver PTX results in fewer complications and shorter […]
Cook Medical India Opens New Facility in Chennai
CHENNAI, India–(BUSINESS WIRE)–Cook Medical India celebrated its new office opening. The company has shifted to a modern, 20,000-square foot office at Kochar Jade in Guindy, Chennai. It is located at the city’s major business district with easy access to land and air freight stations. India is an important part of […]
Beacon® Tip Catheters Available in Europe Again
LIMERICK, Ireland–(BUSINESS WIRE)–Cook Medical is pleased to announce that Beacon® Tip Catheters are once again available to physicians in Europe. As of September 2019, the catheters are available in the UK, Ireland, Norway, Sweden, Finland, Denmark, Germany, Poland, the Netherlands, Luxembourg, Belgium, France, Switzerland, Austria, Hungary, Spain and Italy. Cook is […]
First US Patient Treated with Cook Medical’s Recently Approved Zenith® Dissection Endovascular System
BLOOMINGTON, Ind.–(BUSINESS WIRE)–Following the recent FDA approval of Cook Medical’s Zenith Dissection Endovascular System, Cooper University Health Care in Camden, New Jersey, has treated the first patient in the US with the device as part of Cook’s US commercial launch. “We are committed to helping patients by developing a variety […]
Cook Medical Receives U.S. FDA Approval for Aortic Dissection Device
BLOOMINGTON, Ind.–(BUSINESS WIRE)–Today, Cook Medical announced its recent approval from the U.S. FDA for its Zenith Dissection Endovascular System. The system, consisting of a proximal stent-graft component and a distal bare stent component, provides physicians a less invasive alternative to open surgery for repair of Type B dissections of the […]