WASHINGTON—The Society for Cardiovascular Angiography & Interventions (SCAI) and Cardiovascular Research Technologies (CRT) today announce a new collaboration to elevate the field of interventional cardiology through shared education programming, legislative activities, and future research initiatives. As part of the collaboration, CRT is offering SCAI members discounted registration for CRT 2026, […]
Tag: CRT
JenaValve’s Trilogy THV System Highlighted at CRT 2024
IRVINE, Calif., March 19, 2024 (GLOBE NEWSWIRE) — JenaValve Technology, Inc., developer and manufacturer of the Trilogy™ Transcatheter Heart Valve (THV) System, today announced highlights from the Cardiovascular Research Technologies (CRT) Conference 2024. JenaValve’s Trilogy THV system, a TAVR system designed to treat patients with symptomatic, severe aortic regurgitation (ssAR), and symptomatic, severe aortic stenosis (ssAS), was featured in multiple scientific sessions highlighting the significant unmet need for devices to treat ssAR.
New late-breaking data from Medtronic Evolut™ Low Risk Trial demonstrate strong clinical and cost-effectiveness benefits of TAVR platform
CRT 2024: Evolut TAVR is a safe, effective, and economically cost-effective treatment option for low-risk severe aortic stenosis patients compared to surgery up to four years DUBLIN, March 11, 2024 — Medtronic plc, a global leader in healthcare technology, today announced two late-breaking data presentations on four-year outcomes from […]
4C Medical’s Novel Mitral Regurgitation Therapy to be Highlighted at CRT 2019 Meeting
BROOKLYN PARK, Minn., Feb. 25, 2019 /PRNewswire/ — 4C Medical Technologies, Inc. (4C Medical), a developer of minimally invasive technologies for structural heart disease, today announced that its AltaValve™, a transcatheter mitral valve replacement (TMVR) technology, will be highlighted in the following presentations at the Cardiovascular Research Technologies (CRT) meeting held March 2-5, 2019 in Washington, […]
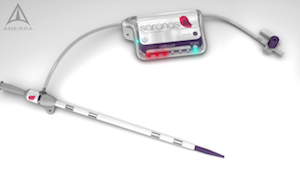
Saranas’ Novel Bleed Monitoring System Receives Recognition in Cardiovascular Research Technologies 2018 Competition
HOUSTON–(BUSINESS WIRE)– Saranas, a medical device company with a cutting-edge technology for real-time detection and monitoring of internal bleeding during endovascular procedures, placed first runner-up at the Cardiovascular Research Technologies (CRT) competition on March 6, 2018, in Washington, D.C. “We are extremely honored that our Early Bird™ Bleed Monitoring System has been recognized […]
EBR Systems, Inc. Initiates Global Trial of World’s Only Wireless CRT Pacing Technology
SUNNYVALE, Calif.–(BUSINESS WIRE)–EBR Systems, Inc., developer of the world’s only wireless cardiac pacing system for heart failure, today announced enrollment of the first patients in the global SOLVE-CRT (Stimulation of the Left Ventricular Endocardium for Cardiac Resynchronization Therapy) clinical trial. The first patients were enrolled by Prof. Dr. Christian Butter […]
Fewer Leads, Fewer Complications: BIOTRONIK US Launches Proven DX Technology for Heart Failure Patients
LAKE OSWEGO, Ore., July 19, 2017 /PRNewswire/ — BIOTRONIK today announced FDA approval and availability of the Intica DX and Intica cardiac resynchronization therapy (CRT)-DX implantable cardioverter defibrillator (ICD) systems. The launch of Intica CRT-DX extends the proven benefits of BIOTRONIK’s DX technology to heart failure patients. DX eliminates the need for an atrial […]
EBR Systems Awarded Favorite Innovation For Wise CRT At 2017 EHRA Europace Cardiostim Congress
SUNNYVALE, Calif.–(BUSINESS WIRE)–EBR Systems, Inc., developer of the world’s smallest, wireless, implantable device for cardiac pacing, today announced that WiSE CRT received the Favorite Innovation Award for its second-generation system at the 2017 EHRA Europace Cardiostim congress in Vienna, Austria. Cardiostim Innovation Awards are selected by an international panel of […]
Medtronic (MDT) Release: New Data Reveal Medtronic CRT Devices Improve Therapy Delivery And Reduce Healthcare Costs
DUBLIN and VIENNA – June 20, 2017 – Medtronic plc (NSYE: MDT) Medtronic Press Release: today announced new data showing that use of its cardiac resynchronization therapy (CRT) devices – with its proprietary AdaptivCRT(TM) and EffectiveCRT(TM) algorithms – results in lower healthcare system costs, and improves therapy delivery in heart […]