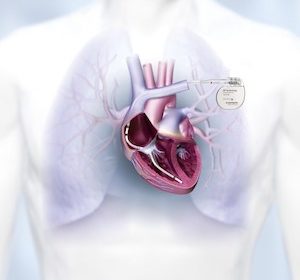
First Enrollments Underway for BIO-AffectDX Trial Evaluating Advanced, Two-Lead CRT-DX System LAKE OSWEGO, Ore., July 30, 2021 /PRNewswire/ — In association with Heart Rhythm 2021, BIOTRONIK today announced first enrollments in the landmark BIO-AffectDX trial, designed to evaluate clinical outcomes of cardiac resynchronization therapy (CRT) in patients with heart failure (HF) and atrial fibrillation (AF). BIO-AffectDX is a multi-center, observational study that will enroll HF […]
Tag: CRT-D
Abbott Receives FDA Approval for New Heart Rhythm Devices Featuring Bluetooth Connectivity and Continuous Remote Monitoring
ABBOTT PARK, Ill., July 6, 2020 /PRNewswire/ — Abbott (NYSE: ABT) today announced that the U.S. Food and Drug Administration (FDA) has approved the company’s next-generation Gallant™ implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy defibrillator (CRT-D) devices. The devices bring new benefits to patients with heart rhythm disorders, including […]
Medtronic (MDT) First To Receive Health Canada Licence For MR-Conditional Cardiac Resynchronization Therapy-Defibrillators
BRAMPTON, Ontario, July 26, 2017 (GLOBE NEWSWIRE) — Medtronic Canada, a subsidiary of Medtronic plc, today announced it has received a Health Canada licence and is launching its first and only magnetic resonance imaging (MRI) conditional cardiac resynchronization therapy defibrillators (CRT-Ds) for heart failure. Heart failure causes or contributes to […]