MINNEAPOLIS, Jan. 10, 2022 (GLOBE NEWSWIRE) — CVRx, Inc. (“CVRx”), a commercial-stage medical device company focused on developing, manufacturing and commercializing innovative neuromodulation solutions for patients with cardiovascular diseases, today announced certain preliminary unaudited fourth quarter and full year 2021 revenue results, and provided a 2022 business outlook. Fourth Quarter […]
Tag: CVRx
CVRx Reports Third Quarter 2021 Financial and Operating Results
Third Quarter 2021 Revenue of $3.4 million, a 241% Increase Over Prior Year MINNEAPOLIS, Nov. 04, 2021 (GLOBE NEWSWIRE) — CVRx, Inc. (NASDAQ: CVRX) (“CVRx”), a commercial-stage medical device company focused on developing, manufacturing and commercializing innovative and minimally invasive neuromodulation solutions for patients with cardiovascular diseases, today announced its […]
CVRx Added to Russell 2000® and 3000® Indexes
MINNEAPOLIS, Sept. 17, 2021 (GLOBE NEWSWIRE) — CVRx, Inc. (NASDAQ: CVRX) (“CVRx”), a commercial-stage medical device company focused on developing, manufacturing and commercializing innovative and minimally invasive neuromodulation solutions for patients with cardiovascular diseases, today announced it will be added as a member of the Russell 2000® and 3000® Indexes, effective after […]
CVRx Reports Second Quarter 2021 Financial and Operating Results
Second Quarter 2021 Revenue of $3.1 Million, a 150% Increase Over Prior Year MINNEAPOLIS, Aug. 04, 2021 (GLOBE NEWSWIRE) — CVRx, Inc. (NASDAQ: CVRX) (“CVRx”), a commercial-stage medical device company focused on developing, manufacturing and commercializing innovative and minimally invasive neuromodulation solutions for patients with cardiovascular diseases, today announced its […]
Treo Ventures Announces the Successful IPO of its Portfolio Company CVRx® (NSDQ: CVRX)
SANTA CLARA, Calif. and DUBLIN, July 6, 2021 /PRNewswire/ — Treo Ventures portfolio company CVRx, Inc. announced the upsized pricing of its initial public offering on the Nasdaq Global Select Market. Treo led the pre-IPO round in July 2020 with Treo General Partner, Mudit K. Jain, PhD, joining the CVRx Board. CVRx is a commercial-stage medical technology company […]
CVRx, Inc. Announces Upsized Pricing of Initial Public Offering
MINNEAPOLIS, June 29, 2021 (GLOBE NEWSWIRE) — CVRx, Inc. (“CVRx”), a commercial-stage medical device company focused on developing, manufacturing and commercializing innovative and minimally invasive neuromodulation solutions for patients with cardiovascular diseases, announced today the upsize and pricing of its initial public offering of 7,000,000 shares of its common stock […]
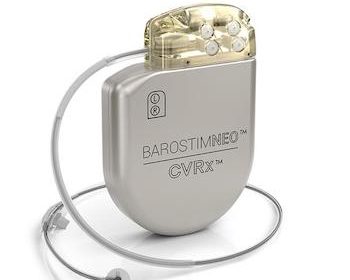
CVRx® Announces First Clinical Procedure with a New Ultrasound-Guided Implant Approach, the Latest Advancement in Barostim™ for the Treatment of Heart Failure Symptoms
MINNEAPOLIS, June 10, 2021 (GLOBE NEWSWIRE) — CVRx®, developer of the world’s first FDA-approved neuromodulation device to treat the symptoms of heart failure (HF), announced the completion of the first clinical procedure with the company’s new lead implantation approach. The novel ultrasound-guided technique is the latest advancement of CVRx’s Barostim™ Baroreflex Activation […]
CVRx® Appoints New Chief Marketing Officer and Vice President of European Sales and Marketing
Paul Verrastro Named CMO and Thomas Hengsteler Named VP of European Sales and Marketing MINNEAPOLIS, Jan. 05, 2021 (GLOBE NEWSWIRE) — CVRx®, developer of the world’s first FDA-approved neuromodulation device to treat the symptoms of heart failure (HF), announced that it is expanding its senior leadership team by adding two medical […]
CVRx® BAROSTIM Therapy Receives CMS Approval for Transitional Pass-Through Payment Status
Incremental Reimbursement in Outpatient Setting Will Expand Availability of the Breakthrough BAROSTIM Therapy Heart Failure Treatment MINNEAPOLIS, Dec. 03, 2020 (GLOBE NEWSWIRE) — CVRx®, developer of the world’s first FDA-approved neuromodulation device to treat the symptoms of heart failure (HF), announced that the Centers for Medicare and Medicaid Services (CMS) granted […]
CVRx® Announces New CFO and Acceleration of Barostim Therapy Commercialization Efforts
MINNEAPOLIS, Nov. 18, 2020 (GLOBE NEWSWIRE) — CVRx, Inc., developer of FDA-approved Barostim Therapy to treat chronic heart failure (HF), announced a CFO transition as part of the company’s long-term growth plan. Jared Oasheim, who joined CVRx in 2015, was promoted from Vice President of Finance to Chief Financial Officer (CFO). He […]