ABBOTT PARK, Ill., Oct. 30, 2017 /PRNewswire/ — Abbott today announced it received CE Mark for XIENCE Sierra, the newest generation of the company’s gold-standard XIENCE everolimus-eluting coronary stent system. CE Mark allows sale of the device in the European Union and other countries that recognize CE Mark. Advances in this generation […]
Tag: DES
Boston Scientific presents 3 year DES MAJESTIC data
Boston Scientific shared the 3-year MAJESTIC DES data online at CardioVascular and Interventional Radiology MAJESTIC 3-Year Data Published for Boston Scientific’s Eluvia DES, by Endovascular Today October 11, 2017—The 3-year results of the MAJESTIC first-in-human study of the Eluvia paclitaxel-eluting vascular stent system (Boston Scientific) for treating femoropopliteal artery lesions were published […]
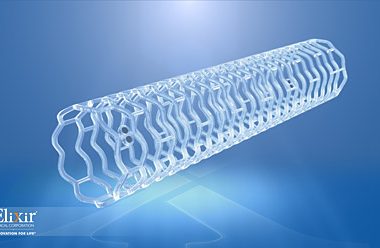
Elixir Medical Corporation to Unveil Game-Changing DynamXTM Metallic Stent at the 29th Transcatheter Cardiovascular Therapeutics Conference
MILPITAS, Calif.–(BUSINESS WIRE)–Elixir Medical Corporation, a leader in the development of breakthrough adaptive remodeling technologies designed to mimic the normal arterial function after cardiovascular and peripheral vascular disease intervention, announced today it will unveil a game-changing metallic drug eluting stent (DES) platform at this year’s Transcatheter Cardiovascular Therapeutics (TCT) conference […]
BIOTRONIK Announces First Enrollments to BIOVITESSE Trial
(PresseBox) – BIOTRONIK has announced the start of enrollment of a coronary stent trial aiming at assessing the safety and clinical performance of a new coronary stent in de novo coronary artery lesions. On September 28, first Dr. Marco Moccetti, Cardiocentro Ticino, Lugano, Switzerland, and later on the same day Dr. Lorenz Raeber, University […]
ESC Congress 2017: BIOTRONIK’s Orsiro Drug-Eluting Stent Outperforms Xience in BIOFLOW-V Trial
Landmark Data Published by The Lancet Demonstrates Statistically Significant Lower Event Rates with Orsiro BARCELONA, Spain, August 28, 2017 – BIOTRONIK today announced data from the BIOFLOW-V randomized trial comparing Orsiro1 and Xience2 drug-eluting stents (DES) with 12-month target lesion failure (TLF) as the primary endpoint proving non-inferiority. Results presented at the […]
Angiolite BTK, below the knee sirolimus-eluting stent, changed CE Mark Approval
(BTK), improving blood flow in severe claudication and critical limb ischaemia. Angiolite BTK design has been specifically elaborated for drug eluting stent and benefits from iVascular proprietary coating nanotechnology that yields a multilayer thin coating with optimal kinetics. “Having the right bail-out options, when performing BTK angioplasty, is extremely important in […]
Stent coated with Viagra may help prevent blood clots
Stent coated with Viagra may help prevent blood clots By AMERICAN HEART ASSOCIATION NEWS Coating heart stents with the erectile dysfunction drug Viagra, known by the generic name sildenafil, could one day reduce blood clots and re-narrowing of arteries, according to new research. Stents prop open heart arteries to help […]
Onyx DES launching this week in Japan
Medtronic will add Onyx to the Resolute stents available in Japan (Integrity has been selling there since 2012). Resolute Onyx drug-eluting stent launches in Japan JULY 7, 2017 BY SARAH FAULKNER Medtronic (NYSE:MDT) said this week that it’s Resolute Onyx drug-eluting stent will launch in Japan on July 10th. The drug-eluting stent has […]
Medtronic Launches Resolute Onyx (TM) Drug-Eluting Stent in US Following FDA Approval Becoming First DES Available in 4.5mm and 5mm Sizes
By GlobeNewswire Engineered for Exceptional Deliverability, Advanced DES Technology Features Thinner Struts, Enhanced Visibility and the Broadest Size Matrix in the U.S. DUBLIN – May 1, 2017 -Medtronic plc (NYSE:MDT) today announced the U.S. Food and Drug Administration (FDA) approval and U.S. launch of the Resolute Onyx(TM) Drug-Eluting Stent (DES). […]