ORLANDO, Fla., March 10, 2018 /PRNewswire/ — The Janssen Pharmaceutical Companies of Johnson & Johnson today announced late-breaking results of a new home-based clinical trial showing that a wearable continuous electrocardiogram (ECG) monitoring patch can identify people with asymptomatic atrial fibrillation (AFib) earlier and more efficiently than routine care. One-year findings from […]
Tag: ECG
HeartSciences Showcases High Sensitivity ECG at 2018 Arab Health Meeting
SOUTHLAKE, Texas–(BUSINESS WIRE)– HeartSciences, an innovative medical device company, is proud to announce its participation as an exhibitor at the upcoming 2018 Arab Health show at the Dubai International Convention and Exhibition Centre in Dubai, UAE, 29 January through 01 February, 2018. HeartSciences is advancing the field of electrocardiology in combining […]
iMedrix Announces General Availability of KardioScreen, a CE certified, Connected ECG Device for Universal Use
BANGALORE, January 5, 2018 /PRNewswire/ — iMedrix, a Silicon Valley and Bangalore-based mHealth start-up, announced the general availability of its product KardioScreen, a CE certified mobile/portable hospital grade digital ECG. KardioScreen is the first truly mobile, connected ECG solution that can be applied universally: field screening, point of care, ambulance, Cardiac ICU and […]
Cardiac Insight Secures First Cardiology Customers for Its Wearable ECG Sensor, Cardea SOLO™
KIRKLAND, Wash.–(BUSINESS WIRE)– Cardiac Insight, a U.S. developer of wearable medical devices and diagnostic software, announced today that the company has secured its first cardiology customers for its wearable ECG sensor device, Cardea SOLO, in Texas and several other states. Early physician and patient experiences with Cardea SOLO are confirming the […]
Imricor and MiRTLE Medical Announce Joint Development Agreement to Deliver 12-Lead ECG for Real-Time MRI-Guided Cardiac Ablations
MINNEAPOLIS–(BUSINESS WIRE)–Imricor and MiRTLE Medical announce a joint development agreement to fully integrate MiRTLE’s MR compatible 12-lead ECG system with Imricor’s Advantage-MR™ EP Recorder/Stimulator System. This integration, along with Imricor’s MR compatible catheters, will allow physicians to perform complex cardiac ablations, such as for treating ventricular tachycardia and atrial fibrillation, under real-time MRI-guidance. […]
HeartSciences Launches Breakthrough ECG Technology at the Canadian Cardiovascular Congress
MyoVista® hsECG™ Utilizes Wavelet Technology to Detect Cardiac Diastolic Dysfunction SOUTHLAKE, Texas, Oct. 19, 2017 /PRNewswire-USNewswire/ — HeartSciences announced today that the company will launch its MyoVista® high sensitivity electrocardiograph (hsECG™) Testing Device in Canada at the Canadian Cardiovascular Congress (CCC) in Vancouver on October 21-24. The MyoVista hsECG is now available in Canada, where it received Health Canada Medical […]
FDA Grants Marketing Clearance for the Peerbridge Cor™ Multi-channel Remote ECG Monitor
NEW YORK, Oct. 4, 2017 /PRNewswire/ — Peerbridge Health Inc., a Health IT company, announced today that its first product, the Peerbridge Cor™ System — a wireless electrocardiogram (ECG) monitor — has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). The patented Peerbridge Cor has the smallest on-body footprint of any […]
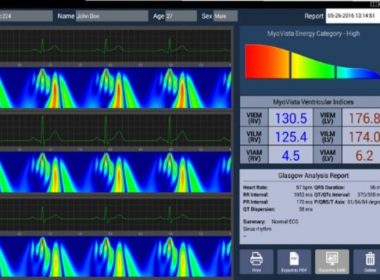
HeartSciences Announces CE Mark and European Launch of MyoVista® High Sensitivity ECG Device
WESTLAKE, Texas, Aug. 17, 2017 /PRNewswire/ — HeartSciences today announced the European launch of MyoVista® high sensitivity electrocardiograph (hsECG™) Testing Device, developed in response to the global unmet need for effective, low-cost, front-line screening of cardiac disease in both symptomatic and asymptomatic patients. MyoVista measures the heart’s energy during each […]
AI That Sees the Invisible: AliveCor and Mayo Clinic Announce Collaboration to Develop Groundbreaking AI Technology to Help Prevent Sudden Cardiac Death
AliveCor Press Release July 19, 2017 – Mountain View, CA – AliveCor, the leader in FDA-cleared personal electrocardiogram (ECG) technology, today announced a collaboration with Mayo Clinic to develop tools for medical and non-medical personnel to easily screen for long QT syndrome (LQTS) early by combining AliveCor’s AI technology with […]
Cardiac Insight’s “Cardea SOLO” Supports Top Arrhythmia Experts’ Acknowledgement: Wearable Cardiac Monitors Are Effective For Tracking Atrial Fibrillation Following Cardiac Ablation
KIRKLAND, Wash.–(BUSINESS WIRE)–Cardiac Insight, Inc., a U.S. developer of wearable medical devices and diagnostic software, announced today that its FDA-approved Cardea SOLO™ ECG monitoring system is available for diagnosing atrial fibrillation following cardiac ablation. The company’s cardiac monitoring device supports a recent formal consensus statement by experts from 11 leading […]