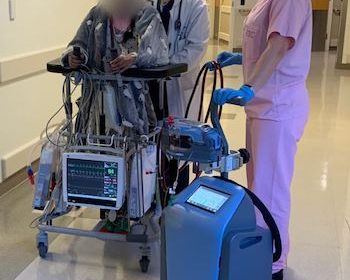
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces the first two patients in the world have been treated with the Abiomed Breethe OXY-1 System, a compact cardiopulmonary bypass system. The advanced ECMO technology pumps, oxygenates, and removes carbon dioxide from blood for patients whose lungs can no longer provide sufficient end organ oxygenation. The […]
Tag: ECMO
FDA Grants 510(k) Clearance for Abiomed’s Innovative Cardiopulmonary Support Technology
DANVERS, Mass.–(BUSINESS WIRE)–The United States Food and Drug Administration (FDA) has granted Abiomed (NASDAQ: ABMD) a 510(k) clearance for an all-in-one, compact cardiopulmonary bypass system called the Abiomed Breethe OXY-1 System™. The ECMO system provides cardiopulmonary bypass support for patients whose lungs can no longer provide sufficient end organ oxygenation. The 510(k) clearance […]
Per FDA Guidance, Medtronic Temporarily Modifies Several Cardiopulmonary Product Indications for Longer Duration Use in ECMO Therapy to Address COVID-19
DUBLIN, May 29, 2020 (GLOBE NEWSWIRE) — During this critical time, Medtronic remains committed to our strategy of delivering critical care products ― designed and indicated for Extracorporeal Membrane Oxygenation (ECMO) ― to treat patients around the world. Following guidance issued by the U.S. Food and Drug Administration (FDA) on April 6, […]