BOULDER, Colo., Nov. 10, 2025 /PRNewswire/ — Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company developing novel therapeutics for muscular dystrophies and serious cardiac conditions, today announced the appointment of Michael Nofi, as Chief…
Tag: Edgewise Therapeutics
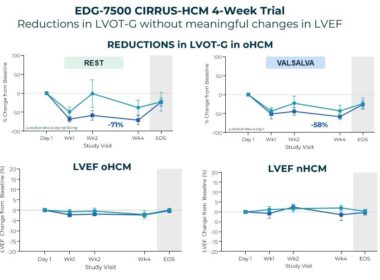
Edgewise Therapeutics Announces Positive Top-Line Results from Phase 2 CIRRUS-HCM Four-Week Trial of EDG-7500 in Hypertrophic Cardiomyopathy (HCM)
– Phase 2 trial of EDG-7500 demonstrated rapid and clinically meaningful reductions in LVOT gradients in participants with obstructive HCM – – Four-week treatment with EDG-7500 demonstrated substantial improvements in measures of feel and function, reductions in key cardiac biomarkers and…
Edgewise Therapeutics Reports Third Quarter 2024 Financial Results and Recent Business Highlights
November 07, 2024 08:00 AM Eastern Standard Time BOULDER, Colo.–(BUSINESS WIRE)–Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today reported financial results for the third quarter of 2024 and recent business highlights. “Based on the strength of clinical and preclinical data to-date, we are treating patients with […]
Edgewise Therapeutics to Host Webcast Event to Discuss Top-Line Data from Phase 1 trial in Healthy Subjects and Phase 2 CIRRUS-HCM Trial in Patients with Obstructive Hypertrophic Cardiomyopathy (HCM) on Thursday, September 19 at 8:30 am Eastern Time
BOULDER, Colo.–(BUSINESS WIRE)–Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that members of the management team will hold a live webcast to discuss top-line data of EDG-7500 from the Phase 1 trial in healthy subjects and the single-dose arm of the Phase 2 CIRRUS-HCM trial in […]
Edgewise Therapeutics Announces Upcoming Podium Presentation at the American College of Cardiology's 2024 Annual Scientific Session
BOULDER, Colo.–(BUSINESS WIRE)–Edgewise Therapeutics, Inc. (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that the Company will present data on EDG-7500 at the American College of Cardiology’s Annual Scientific Session (ACC.24). EDG-7500 is a first-in-class oral, selective, cardiac sarcomere modulator, specifically designed to slow early contraction velocity and address impaired cardiac relaxation associated with HCM and other diseases of diastolic dysfunc
Edgewise Therapeutics Reports Third Quarter 2023 Financial Results and Recent Business Highlights
BOULDER, Colo.–(BUSINESS WIRE)–Edgewise Therapeutics, Inc. (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today reported financial results for the third quarter of 2023 and recent business highlights. “I’m thrilled with the progress we’ve made in 2023 across our skeletal and cardiovascular programs,” said Kevin Koch, Ph.D., President and Chief Executive Officer of Edgewise. “Most recently, we started enrolling individuals in two important studies: GRAND CANYON, a global p
Edgewise Therapeutics Begins Dosing First-in-Human Phase 1 Trial of EDG-7500, its Lead Clinical Candidate for Hypertrophic Cardiomyopathy (HCM) and Other Serious Diseases of Cardiac Diastolic Dysfunction
BOULDER, Colo.–(BUSINESS WIRE)–Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced initial dosing in a Phase 1 trial of EDG-7500. EDG-7500 is a first-in-class oral, selective, cardiac sarcomere modulator, specifically designed to slow early contraction velocity and address impaired cardiac relaxation associated with HCM and other […]
Edgewise Therapeutics Reports Second Quarter 2023 Financial Results and Recent Business Highlights
BOULDER, Colo.–(BUSINESS WIRE)–Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a clinical-stage biopharmaceutical company focused on the discovery, development, and commercialization of innovative treatments for severe, genetic neuromuscular and cardiac disorders for which there is significant unmet medical need, today reported financial results for the second quarter of 2023 and recent business highlights. […]