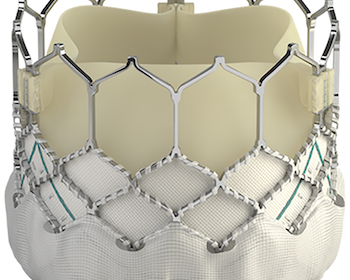
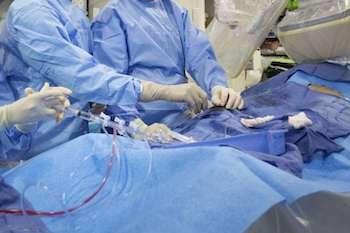
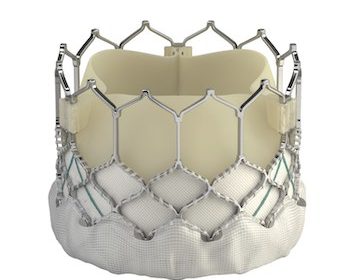
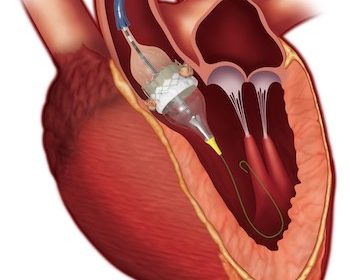
IRVINE, Calif., July 22, 2021 /PRNewswire/ — Edwards Lifesciences (NYSE: EW) today announced that clinical and economic results from the 3M (multidisciplinary, multimodality, but minimalist) Transcatheter Aortic Valve Replacement (TAVR) Economic Study and the PARTNER 3 Bicuspid Registry for SAPIEN 3 TAVR Study were presented at TVT 2021: The Structural Heart Summit. The 3M-TAVR study demonstrated positive […]
Tag: Edwards
Edwards’ KONECT RESILIA Aortic Valved Conduit Receives FDA Approval For Complex Aortic Valve Surgeries
IRVINE, Calif., July 15, 2020 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced it received approval from the U.S. Food and Drug Administration (FDA) for the KONECT RESILIA aortic valved conduit (AVC), the first ready-to-implant solution for bio-Bentall procedures, […]
Edwards SAPIEN 3 Transcatheter Heart Valve Receives Approval In China
IRVINE, Calif., June 8, 2020 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced that it has received Chinese regulatory approval for the Edwards SAPIEN 3 transcatheter heart valve for the treatment of patients suffering from severe, symptomatic aortic […]
Edwards SAPIEN 3 Valve Shows Excellent Results At 2 Years
IRVINE, Calif., March 29, 2020 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced 2-year results of the randomized PARTNER 3 trial comparing treatment with the SAPIEN 3 valve to surgery in patients with severe symptomatic aortic stenosis (AS) at […]
Edwards Pauses Enrollments In Pivotal Mitral, Tricuspid Trials In Response To Hospitals’ Focus On COVID-19
IRVINE, Calif., March 28, 2020 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced it will temporarily pause new enrollments in its active pivotal clinical trials of transcatheter mitral and tricuspid therapies, in response to the urgent COVID-19 response […]
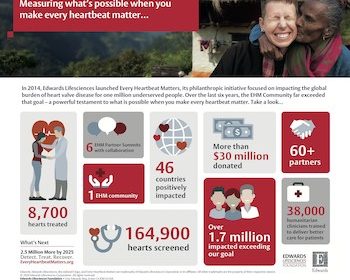
Edwards Lifesciences’ Every Heartbeat Matters Philanthropic Initiative Expanding To Reach More Patients
IRVINE, Calif., Feb. 21, 2020 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced a new goal for its philanthropic initiative, Every Heartbeat Matters (EHM): to improve the lives of 2.5 million additional underserved structural heart and critical care patients by the end […]
Edwards SAPIEN 3 TAVI Receives Expanded Approval In Europe
Superior TAVI Valve Indicated for All Patients Diagnosed with Aortic Stenosis IRVINE, Calif., Nov. 6, 2019 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced that it has received CE Mark to expand use of the Edwards SAPIEN 3 […]
Edwards SAPIEN 3 TAVR Receives FDA Approval For Low-Risk Patients
IRVINE, Calif., Aug. 16, 2019 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced U.S. Food and Drug Administration (FDA) approval to expand use of the Edwards SAPIEN 3 and SAPIEN 3 Ultra transcatheter heart valve systems to the treatment […]
Edwards’ SAPIEN 3 Ultra Transcatheter Heart Valve Receives FDA Approval
IRVINE, Calif., Dec. 28, 2018 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced that the SAPIEN 3 Ultra system has received U.S. Food and Drug Administration (FDA) approval for transcatheter aortic valve replacement in severe, symptomatic aortic stenosis patients […]
Edwards Comments On CMS Initiation Of National Coverage Analysis For TAVR
IRVINE, Calif., June 28, 2018 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today commented on the announcement by the U.S. Centers for Medicare and Medicaid Services (CMS) about a National Coverage Analysis (NCA) for transcatheter aortic valve replacement (TAVR): CMS […]