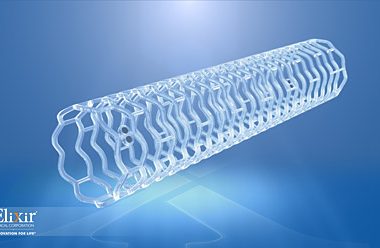
Successful DynamX Bioadaptor Live Case Presented at Singapore Live MILPITAS, Calif.–(BUSINESS WIRE)–Elixir Medical, a developer of innovative, drug-eluting cardiovascular devices, today announced the 24-month clinical results for the DynamX™ Coronary Bioadaptor System, the first drug-eluting coronary artery implant that adapts to vessel physiology. The results were presented at the 30th Annual Live […]
Tag: Elixir
Elixir Medical Announces Outstanding 12-Month Data for DynamX Coronary Bioadaptor System, Demonstrating No Target Vessel Revascularization, No Thrombosis and Positive Adaptive Remodeling of the Artery
PARIS–(BUSINESS WIRE)–Elixir Medical, a developer of innovative, drug-eluting cardiovascular devices, today announced outstanding 12-month data for the DynamX™ Coronary Bioadaptor System, the first coronary artery implant that adapts to vessel physiology. Results will be presented live tomorrow at 14:12 CEST on the Main Arena channel of the PCR e-Course as part […]
Elixir Medical Corporation to Unveil Game-Changing DynamXTM Metallic Stent at the 29th Transcatheter Cardiovascular Therapeutics Conference
MILPITAS, Calif.–(BUSINESS WIRE)–Elixir Medical Corporation, a leader in the development of breakthrough adaptive remodeling technologies designed to mimic the normal arterial function after cardiovascular and peripheral vascular disease intervention, announced today it will unveil a game-changing metallic drug eluting stent (DES) platform at this year’s Transcatheter Cardiovascular Therapeutics (TCT) conference […]
Elixir Medical Announces Outstanding 5-Year Clinical Data For CE Mark-Approved Desolve Novolimus Eluting Bioresorbable Coronary Scaffold System
MILPITAS, Calif.–(BUSINESS WIRE)–Elixir Medical Corporation, a developer of products that combine state-of-the-art medical devices with advanced pharmaceuticals, today announced excellent 5-year clinical data from the DESolve Nx international pivotal clinical trial for the CE Mark-approved, fully bioresorbable DESolve® Novolimus Eluting Coronary Scaffold System. Stefan Verheye, MD, PhD, ZNA Middleheim Hospital, […]