Company Also Receives European CE Mark Approval for Novel Stroke Technology Designed for Difficult to Extract Blood Clots IRVINE, Calif., Dec. 10, 2018 — Johnson & Johnson Medical Devices Companies* today announced that its CERENOVUS business has launched the single largest global registry, the EXCELLENT Registry, to collect and analyze […]
Tag: Embotrap II
Capital Health First in New Jersey to Offer FDA-Approved EMBOTRAP II Stent Retriever for Ischemic Stroke Patients
TRENTON, N.J., Aug. 20, 2018 /PRNewswire/ — Capital Health is the first hospital in New Jersey, and among the first in the United States, to use the new EMBOTRAP II Revascularization Device since its recent approval from the US Food and Drug Administration (FDA) in July. As part of its Comprehensive Stroke Center, neurosurgeons […]
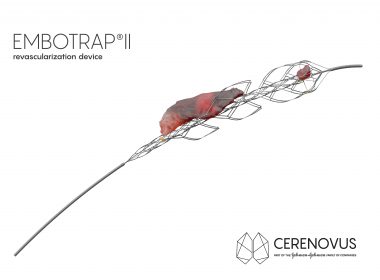
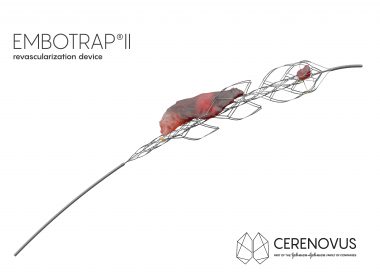
First Ischemic Stroke Patients Treated With EMBOTRAP II Revascularization Device Since Commercial Availability In U.S.
SAN FRANCISCO, July 24, 2018 /PRNewswire/ — CERENOVUS, part of the Johnson & Johnson Medical Devices Companies, announced the first patients have been treated with its new EMBOTRAP II Revascularization Device since it became commercially available in the U.S. EMBOTRAP II is a next generation stent retriever used to capture and remove life-threatening blood clots […]