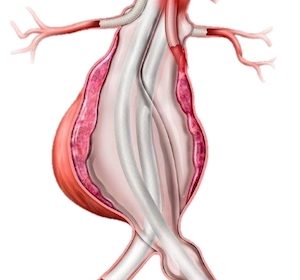
Investigational EVAS System Designed for Patients with Complex Abdominal Aortic Aneurysm IRVINE, Calif.–(BUSINESS WIRE)–Endologix LLC, a leader in the treatment of vascular disease, today announced the company’s ChEVAS™ (Chimney EndoVascular Aneurysm Sealing) System has been granted a Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA). The ChEVAS […]
Tag: Endologix
Endologix LLC Announces Launch of ALTO® Abdominal Stent Graft System in Canada and Argentina
Company Expands Global Reach with Latest Endovascular Aneurysm Repair (EVAR) Technology IRVINE, Calif.–(BUSINESS WIRE)–Endologix LLC, a leader in the treatment of vascular disease, today announced the first implant of its ALTO® Abdominal Stent Graft in Canada following recent approval from Health Canada. ALTO was also recently approved for commercial sale […]
Endologix LLC Announces Acquisition of PQ Bypass, Inc.
Company Expands Vascular Reach to Treat Unmet Needs of Both AAA and PAD Patients IRVINE, Calif.—(BUSINESS WIRE)—April 13, 2021—Endologix LLC, a leader in the treatment of vascular disease, today announced it has completed the acquisition of PQ Bypass, Inc., a privately held medical technology company pioneering a first-of-its-kind technology that addresses […]
Endologix Launches ALTO™ Abdominal Stent Graft System in Europe
First European Commercial Case Completed at East & North Hertfordshire Hospital in England IRVINE, Calif.–(BUSINESS WIRE)—Oct 15, 2020–Furthering its mission to transform the treatment of aortic disorders, Endologix LLC today announced the first implant of its ALTO™ Abdominal Stent Graft, commencing the European commercial release of the recently CE Mark […]
Endologix Announces First Commercial Implant of ALTO Abdominal Stent Graft System Outside of United States
IRVINE, Calif.–(BUSINESS WIRE)–Furthering its mission to transform the treatment of aortic disorders, Endologix, Inc. (OTC: ELGXQ) (“Endologix” or the “Company”) today announced the first implant of its recently approved ALTO® endograft outside of the United States, completed by Andrew Holden, MD, and Andrew Hill, MD, of Auckland City Hospital, Auckland, New […]
Endologix Receives CE Mark for ALTO Abdominal Stent Graft System
IRVINE, Calif.–(BUSINESS WIRE)–Transforming the treatment of aortic disorders, Endologix® Inc. (OTC: ELGXQ) (“Endologix” or the “Company”), today announced that it has received a CE Mark for the ALTO™ Abdominal Stent Graft System (ALTO). “We are very excited to receive a CE Mark for the ALTO system, that has been achieved through […]
Endologix Announces First Commercial Implant of ALTO Abdominal Stent Graft System & Official Start of U.S. Commercial Release
IRVINE, Calif.–(BUSINESS WIRE)–Transforming the treatment of aortic disorders, Endologix® Inc. (OTC: ELGXQ) (“Endologix” or the “Company”), today announced the first commercial implant and the U.S. commercial release of its recently FDA-approved ALTO® endograft for the treatment of abdominal aortic aneurysms (AAA). “ALTO represents a differentiation from traditional endovascular aneurysm repair (EVAR) and, […]
Endologix Enters into an Agreement with Deerfield Partners to Take the Company Private
Commences voluntary Chapter 11 process with access to $30.8 million in DIP financing Files consensual plan of reorganization that would reduce approximately $180 million in debt and provide an approximately $30 million in additional exit financing Confirms launch of Alto this summer, steady production, continued clinical studies, and accelerated pipeline […]
Endologix Announces Departure of CFO and Appointment of Interim CFO
IRVINE, Calif.–(BUSINESS WIRE)–Endologix, Inc. (Nasdaq: ELGX) (“Endologix” or the “Company”), a developer and marketer of innovative treatments for aortic disorders, today announced that Vaseem Mahboob, Chief Financial Officer (CFO), will be departing the Company effective July 1, 2020 to become CFO of a private global healthcare company. Cindy Pinto, Vice […]
Endologix Issues Correction Notice for Ovation iX Abdominal Stent Graft System
IRVINE, Calif.–(BUSINESS WIRE)–Endologix® Inc. (Nasdaq: ELGX) (“Endologix” or the “Company”), a developer and marketer of innovative treatments for aortic disorders, today announced that a correction notice has been issued for the Ovation iX system, that identifies the root cause of polymer leaks. This voluntary action has been classified by the FDA […]