July 24, 2024 04:15 PM Eastern Daylight Time IRVINE, Calif.–(BUSINESS WIRE)–Edwards Lifesciences (NYSE: EW) today announced investments that reflect the company’s deep commitment to advancing patient care through structural heart innovation, addressing large unmet patient needs and supporting sustainable long-term growth. Edwards has entered into an agreement to acquire JenaValve Technology, […]
Tag: Endotronix
Endotronix Receives IDE Approval for the Market Expanding PROACTIVE-HF 2 Clinical Trial
NAPERVILLE, Ill., Oct. 9, 2023 /PRNewswire/ — Endotronix, Inc., a digital health and medical technology company dedicated to advancing the treatment of heart failure (HF), announced it has received Investigational Device Exemption (IDE) approval from the FDA for a subsequent multicenter study, PROACTIVE-HF 2, which will evaluate the company’s Cordella Sensor for pulmonary artery […]
Endotronix Extends Positive Outcomes with 12-month Data Release from the SIRONA 2 Trial for Remote Heart Failure Management Using the Cordella Pulmonary Artery Sensor
The study met secondary study endpoints and long-term sensor use was associated with significant improvements in patient quality-of-life metrics and low event rates for heart failure hospitalization and death LISLE, Ill., May 22, 2023 /PRNewswire/ — Endotronix, Inc., a digital health and medical technology company dedicated to advancing the treatment of heart failure (HF), […]
Endotronix Announces Enrollment Completion of PROACTIVE-HF Pivotal Trial
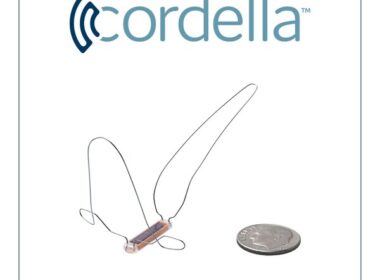
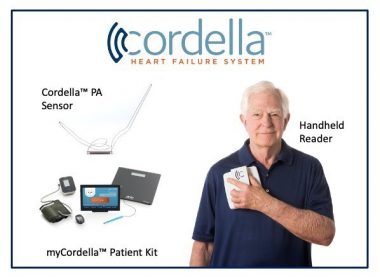
Data to support regulatory submission and accelerate U.S. market access for the Cordella™ Pulmonary Artery (PA) Sensor Cordella™ is the first and only patient management platform to provide both critical PA pressure data, using an implanted sensor, and noninvasive vitals (blood pressure, heart rate, and weight) for comprehensive clinical management delivered in […]
Endotronix Celebrates 400th Implant of Cordella™ Pulmonary Artery Pressure Sensor for Proactive, Remote Heart Failure Management
Company also announces publication of the PROACTIVE-HF trial design rationale, the first global IDE trial for PA pressure-guided heart failure management, in the Journal of Cardiac Failure LISLE, Ill., Sept. 30, 2022 /PRNewswire/ — Endotronix, Inc., a privately-held digital health and medical technology company dedicated to advancing the treatment of heart failure (HF), today announced […]
Endotronix Announces Development Agreement for a New Expanded U.S. Headquarters in Naperville, Illinois
Company is constructing a new commercial manufacturing facility and anticipates expanding its workforce to support the launch of its comprehensive patient management platform for heart failure LISLE, Ill., July 19, 2022 /PRNewswire/ — Endotronix, Inc., a digital health and medical technology company dedicated to advancing the treatment of heart failure (HF), today announced plans […]
Endotronix Announces Positive Data from SIRONA 2 in Late-Breaking Clinical Trial Session
Study met all primary safety and efficacy endpoints for the Cordella™ PA Pressure Sensor System for the treatment of NYHA class III heart failure patients LISLE, Ill., May 23, 2022 /PRNewswire/ — Endotronix, Inc., a digital health and medical technology company dedicated to advancing the treatment of heart failure (HF), today announced positive data […]
Endotronix Announces FDA Approval for PROACTIVE-HF Pivotal Trial Design Change to Single-Arm Study
Amended study design provides immediate access and clinical benefits for all patients and becomes the first global IDE study for PA pressure-guided heart failure management LISLE, Ill., Jan. 5, 2022 /PRNewswire/ — Endotronix, Inc., a digital health and medical technology company dedicated to advancing the treatment of heart failure (HF), today announced the […]
Endotronix Celebrates 100th Implant Of The Cordella™ Pulmonary Artery Pressure Sensor For Proactive, Remote Management Of Heart Failure
Milestone achieved as company prepares for next stage of commercial growth and expansion LISLE, Ill., March 23, 2021 /PRNewswire/ — Endotronix, Inc., a digital health and medical technology company dedicated to advancing the treatment of heart failure, today announced the 100th global implant of the Cordella Pulmonary Artery Pressure Sensor (Cordella Sensor) […]
Endotronix Appoints Industry Veteran Dan Dearen to Board of Directors
Expands company’s financial expertise during time of growth in advance of commercial launch LISLE, Ill., March 2, 2021 /PRNewswire/ — Endotronix, Inc., a digital health and medical technology company dedicated to advancing the treatment of heart failure (HF), today announced the appointment of seasoned medical device industry executive Dan Dearen to its Board of […]