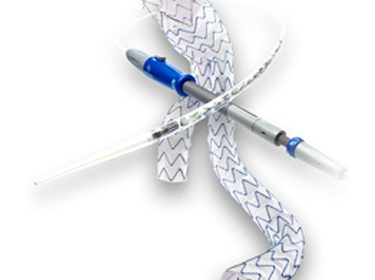
With this labeling approval, Medtronic continues to lead the way in advancing aortic care with clinical evidence for the treatment of ruptured abdominal aortic aneurysms (rAAA). Medtronic plc, a global leader in healthcare technology, today announced it has received U.S. Food and Drug Administration (FDA) labeling approval1 for the Medtronic Endurant™ […]
Tag: Endurant
Medtronic (MDT)’s Stent System Wins FDA OK to Treat Short Neck Anatomies When Used With Heli-FX EndoAnchor System
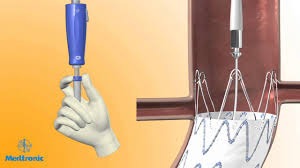
DUBLIN – October 9, 2017 – Medtronic plc (NYSE: MDT) today announced that it has received U.S. Food and Drug Administration (FDA) approval for the Endurant(TM) II/IIs stent graft system to treat abdominal aortic aneurysm (AAA) patients with neck lengths down to 4mm and <=60° infra-renal angulation when used in combination […]
Medtronic (MDT) Shows Off New 5-Year Subset Data From Endurant AAA Stent Graft Trial
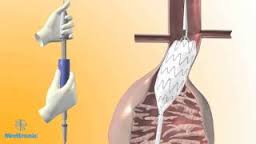
DUBLIN and LAS VEGAS – September 13, 2017 – Medtronic plc (NYSE: MDT) today reported its Endurant® II abdominal aortic aneurysm (AAA) stent graft system continues to demonstrate long-term durability and consistent outcomes in a real-world setting among both male and female patients. The five-year ENGAGE global registry data were presented […]
Five-Year Data from the Medtronic ENGAGE Registry Demonstrate Durable, Consistent, and Proven Outcomes in Real-World Setting
Source: Nasdaq Global Newswire DUBLIN and LONDON – April 26, 2017 – Medtronic plc (NYSE:MDT) today reported its Endurant® II abdominal aortic aneurysm (AAA) stent graft system continues to demonstrate long-term durability and consistent outcomes in a real-world setting. The five-year ENGAGE global registry data were presented for the first time […]