Exo unveils its 10th FDA-cleared AI, delivering instant, on-device Global Longitudinal Strain (GLS) analysis — no cloud, no lag, no guesswork Identify signs of heart failure and cardiotoxicity SANTA CLARA, Calif.–(BUSINESS WIRE)–Exo (pronounced “echo”), the leader in AI-powered, accessible medical imaging, announced its latest FDA 510(k) clearance for Global Longitudinal Strain […]
Tag: Exo
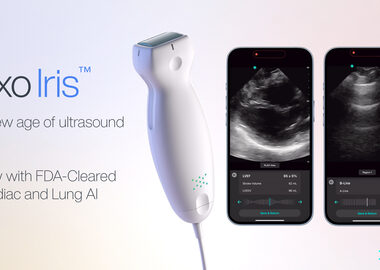
Exo® Launches FDA-Cleared AI on Exo Iris™ to Address Heart Failure
April 23, 2024 08:30 AM Eastern Daylight Time SANTA CLARA, Calif.–(BUSINESS WIRE)–Exo® (pronounced “echo”), a medical imaging software and devices company, today announced its FDA-cleared cardiac and lung artificial intelligence (AI) applications are now available on Exo Iris™, Exo’s high-performance handheld ultrasound device. “Exo’s AI is simple to use, reproducible and objective, and will […]