MOUNTAIN VIEW, Calif.–(BUSINESS WIRE)–Allan Zingeler, Raju Viswanathan, PhD, and Michael Mahoney of Boston Scientific have been awarded the inaugural Thomas J. Fogarty Prize (Fogarty Prize) for the FARAPULSE™ PFA Platform. The award, which includes an unrestricted $100,000 cash prize and a custom-cast bronze medallion, was announced on October 24 at a sold-out black-tie […]
Tag: Farapulse
Boston Scientific receives FDA approval for expanded labeling of FARAPULSE™ Pulsed Field Ablation System
The FARAPULSE PFA System now approved for pulmonary vein and posterior wall ablation in patients with persistent atrial fibrillation MARLBOROUGH, Mass., July 7, 2025 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) has received U.S. Food and Drug Administration (FDA) approval to…
Second phase of ADVANTAGE AF study of FARAPULSE™ Pulsed Field Ablation System meets primary safety and efficacy endpoints
Trial achieves positive results in the treatment of persistent atrial fibrillation MARLBOROUGH, Mass. and SAN DIEGO, April 24, 2025 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced positive 12-month primary endpoint results from the second phase of the ADVANTAGE…
The FARAPULSE™ Pulsed Field Ablation System Demonstrates Superior Efficacy in Patients Treated for Paroxysmal Atrial Fibrillation
New findings from the SINGLE SHOT CHAMPION trial (NCT05534581), presented at EHRA 2025 and published in the New England Journal of Medicine, showed the FARAPULSE™ Pulsed Field Ablation (PFA) System to be superior in reducing atrial arrhythmia (AA) recurrence for treating symptomatic, drug-refractory paroxysmal atrial fibrillation (PAF) compared to the Artic Front Advance™ cardiac cryoablation catheter. […]
Late-breaking data presented at AF Symposium 2025 highlight key Boston Scientific therapies for management of patients with atrial fibrillation
First phase of ADVANTAGE AF clinical trial achieves safety and effectiveness endpoints for treatment of drug-resistant, symptomatic, persistent atrial fibrillation with the FARAPULSE™ Pulsed Field Ablation System Sub-analysis from OPTION clinical trial highlights consistent safety and…
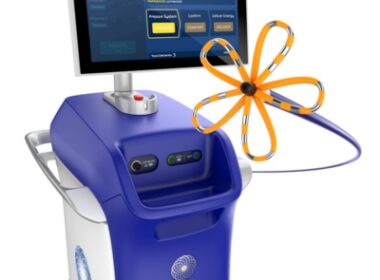
Boston Scientific Launches Next Generation of Cardiac Mapping for the FARAPULSE™ Pulsed Field Ablation System
New FARAWAVE™ NAV Ablation Catheter and FARAVIEW™ Software combine with OPAL HDx™ Mapping System to provide visualization during pulsed field ablation MARLBOROUGH, Mass., Oct. 18, 2024 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced it has received U.S. Food and…
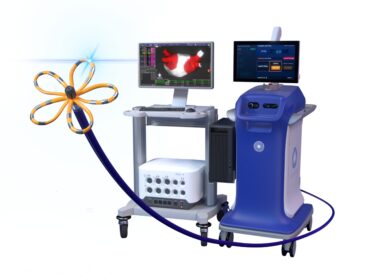
Boston Scientific Receives FDA Approval for FARAPULSE™ Pulsed Field Ablation System
MARLBOROUGH, Mass., Jan. 31, 2024 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) announced it has received U.S. Food and Drug Administration (FDA) approval for the FARAPULSE™ Pulsed Field Ablation (PFA) System. The FARAPULSE PFA System is indicated for the isolation of pulmonary veins in the treatment of drug-refractory, recurrent, symptomatic, paroxysmal (i.e., […]
ADVENT Study of the FARAPULSE™ Pulsed Field Ablation System Meets Primary Efficacy and Safety Endpoints
MARLBOROUGH, Mass. and AMSTERDAM, Aug. 27, 2023 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced positive 12-month results from the pivotal ADVENT clinical trial of the FARAPULSE™ Pulsed Field Ablation (PFA) System*, a nonthermal treatment in which electric fields selectively ablate heart tissue in patients with atrial fibrillation (AF). The study is the first […]
Data at Heart Rhythm 2023 Highlight Key Boston Scientific Therapies
Additional real-world outcomes further demonstrate safety, efficacy and procedural reproducibility of the FARAPULSE™ Pulsed Field Ablation System* Results from global trial of the POLARx™ Cryoablation System* meet safety and effectiveness endpoints MARLBOROUGH, Mass., May 20, 2023 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced data supporting use of the company’s key electrophysiology […]
Boston Scientific Exercises Option to Acquire Farapulse, Inc
Acquisition complements company’s electrophysiology portfolio to include the only commercially available cardiac pulsed field ablation technology MARLBOROUGH, Mass., June 24, 2021 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced it exercised its option to acquire the remaining shares of Farapulse, Inc. The acquisition will complement the existing Boston Scientific electrophysiology portfolio to include […]