Recardio Inc., a late stage clinical-stage life science company developing regenerative therapies for cardiovascular diseases, announced that the FDA concurs with Recardio’s pivotal Heal-MI Phase 3 trial design with Dutogliptin in Acute Myocardial Infarction. SAN FRANCISCO, June 01, 2022 (GLOBE NEWSWIRE) — Recardio’s Phase 2 trial results demonstrated the excellent […]
Tag: FDA
Mezzion Pharma Receives Clear FDA Path Forward for the Approval of Udenafil for Single Ventricle Heart Disease
SEOUL, South Korea and BETHESDA, Md. , May 31, 2022 /PRNewswire/ — Mezzion Pharma Co., Ltd. (Mezzion Pharma) met with the FDA Division of Cardiology and Nephrology (DCN) on Friday, May 27th to discuss with the FDA the path forward for the regulatory approval of udenafil for the treatment of single ventricle heart disease (SVHD) in […]
ŌNŌCOR Receives FDA Clearance for Novel Endovascular Retrieval Technology
The ŌNŌ is intended to simplify bailout procedures in the expanding world of structural heart disease interventions PHILADELPHIA, May 25, 2022 (GLOBE NEWSWIRE) — ŌNŌCOR LLC, a leader in endovascular safety technology, today announced it has received 510(k) U.S. Food and Drug Administration (FDA) clearance for its ŌNŌ retrieval system, […]
FDA Grants Breakthrough Device Designation to Anumana’s ECG Pulmonary Hypertension Early Detection AI Algorithm
Developed in collaboration with Janssen and Mayo Clinic, this technology has the potential to aid physicians in earlier detection of pulmonary hypertension, a progressive, life-threatening disease CAMBRIDGE, Mass.–(BUSINESS WIRE)–Anumana, Inc., an AI-driven health technology company from nference, Inc., today announced that the U.S. Food & Drug Administration (FDA) has granted Breakthrough Device […]
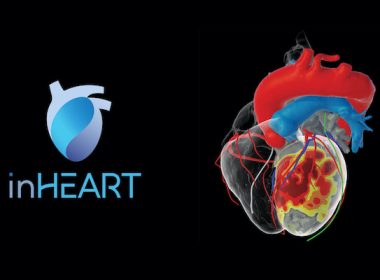
inHEART RECEIVES FDA CLEARANCE FOR NOVEL 3D CARDIAC MODELING SOLUTION
System creates 3D models of the heart with unprecedented anatomical details, allowing physicians to better plan and personalize therapeutic interventions CAMBRIDGE, Mass. and BORDEAUX, France, May 24, 2022 /PRNewswire/ — inHEART, a privately-held medical device company delivering the world’s most sophisticated digital twin of the heart, announced today that it has received FDA 510(k) clearance […]
RapidAI Receives FDA 510(k) Clearance for Pulmonary Embolism Triage & Notification
Clinically driven AI platform extends physicians’ ability to prioritize patients, reduce time to treatment and improve patient outcomes SAN MATEO, Calif.–(BUSINESS WIRE)–RapidAI, the global leader in neurovascular and vascular AI-enhanced clinical decision support and patient workflow, announced today that it has received FDA 510(k) clearance for its Rapid PE Triage & […]
VenoStent Technology Receives Breakthrough Device Designation by FDA
HOUSTON, TX / ACCESSWIRE / May 17, 2022 / VenoStent, Inc., a clinical-stage tissue engineering company developing bioabsorbable perivascular wraps to improve outcomes in the 5 million vascular surgeries performed each year, announces that The Center for Devices and Radiological Health (CDRH) at the Food and Drug Administration (FDA) has granted […]
Vesalio Announces Completion of Enrollment in it’s FDA Enabling Stroke Study
Nashville, TN (May 11, 2022) – Vesalio is excited to announce the completion of patient enrollment for the CLEAR1 FDA IDE study utilizing the NeVa™ thrombectomy platform. This is an important milestone for Vesalio to complete for entering the U.S. neurovascular market to treat patients suffering from acute ischemic stroke (AIS). AIS […]
Medtronic receives FDA approval for latest generation drug-eluting coronary stent system
The Onyx Frontier™ drug-eluting stent offers an innovative delivery system and builds upon the acute performance and clinical data from the Resolute Onyx™ drug-eluting stent DUBLIN, May 13, 2022 /PRNewswire/ — Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced it received U.S. Food and Drug Administration (FDA) approval for the Onyx […]
Boston Scientific Receives FDA Clearance for the EMBOLD™ Fibered Detachable Coil
MARLBOROUGH, Mass., April 28, 2022 – Boston Scientific Corporation (NYSE: BSX) has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the EMBOLD™ Fibered Detachable Coil, which is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature. The first procedure using the device was performed this week […]