Camzyos is the first and only FDA-approved cardiac myosin inhibitor that specifically targets the source of obstructive HCM Approval based on groundbreaking Phase 3 EXPLORER-HCM trial demonstrating benefit in patients receiving Camzyos versus placebo PRINCETON, N.J.–(BUSINESS WIRE)–Bristol Myers Squibb (NYSE: BMY) today announced that the U.S. Food and Drug Administration (FDA) […]
Tag: FDA
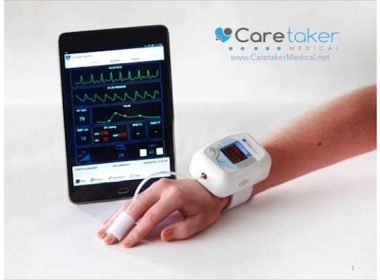
FDA Clears Caretaker Medical’s Wireless Monitor for NonInvasive & Continuous Cardiac Output, Stroke Volume, and Advanced Hemodynamics
VitalStream™, Featuring Patented Pulse Decomposition Analysis™ technology becomes the World’s First Wireless Wearable Monitor for Continuous Blood Pressure and Advanced Hemodynamics – All with a single, easy-to-apply Finger Sensor Caretaker Medical, a digital health leader in continuous “beat by beat” wireless patient monitoring technologies, today announced that it has received […]
TransMedics Receives FDA PMA Approval of OCS™ DCD Heart Indication
ANDOVER, Mass., April 28, 2022 /PRNewswire/ — TransMedics Group, Inc. (“TransMedics”) (Nasdaq: TMDX), a medical technology company that is transforming organ transplant therapy for patients with end-stage lung, heart, and liver failure, today announced that the U.S. Food and Drug Administration (FDA) has granted premarket approval (PMA) of its OCS™ Heart System for […]
BIOTRONIK Announces FDA Approval of Renamic Neo with LiveSupport Software
LAKE OSWEGO, Ore., April 26, 2022 /PRNewswire/ — BIOTRONIK, Inc. announced today it has received U.S. Food and Drug Administration (FDA) approval of Renamic Neo, the company’s state-of-the-art programmer for implanted cardiac rhythm management devices such as ICDs, pacemakers, and implantable cardiac monitors. In addition to its programming functions, Renamic Neo now […]
BENDIT Technologies Receives U.S. Food and Drug Administration 510(k) Clearance of its Bendit 021″ steerable microcatheter
TEL AVIV, Israel, April 18, 2022 /PRNewswire/ — BENDIT Technologies, a company focused on the development of a steerable microcatheter platform, announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Bendit21 microcatheter for treatment in the neuro, peripheral, and coronary vasculature. The clearance was received several […]
Israeli startup Sanolla receives FDA clearance for the world’s first AI-ready infrasound stethoscope
Sanolla’s pioneering technology draws lifesaving medical insights from listening to bodily sounds that cannot be heard by humans The startup’s AI algorithms provide unmatched disease classification for many cardiopulmonary diseases including COPD, pneumonia, asthma, and cardiac morbidities NESHER, Israel, April 11, 2022 /PRNewswire/ — Israeli startup Sanolla, which provides AI-powered primary care diagnostic solutions, announced […]
Triastek Receives FDA IND Clearance for 3D Printed Product of Blockbuster Molecule
NANJING, China, April 7, 2022 /PRNewswire/ — Triastek, Inc. (“Triastek”) recently announced that the United States Food and Drug Administration (FDA) has granted permission to begin clinical studies of its Investigational New Drug (IND) 505(b)(2) application for a 3D printed drug product – T20. It is Triastek’s second product receiving IND clearance from the […]
Arterys Receives Its Eighth FDA Clearance Powered With AI and Releases Its Next-Generation Cardio AI Application
Receives eighth FDA clearance, releases its next-generation Cardio AI application SAN FRANCISCO, April 07, 2022 (GLOBE NEWSWIRE) — Arterys, the world’s leading vendor-neutral AI platform, has announced several new modules to its already robust Cardio AI clinical application and an additional FDA AI clearance based on deep learning. Cardio AI […]
ABBOTT RECEIVES FDA APPROVAL FOR AVEIR™ VR LEADLESS PACEMAKER SYSTEM TO TREAT PATIENTS WITH SLOW HEART RHYTHMS
Abbott’s Aveir single chamber (VR) pacing system is the world’s only leadless pacemaker with a unique mapping capability to assess correct positioning prior to placement Aveir VR has an increased projected battery life that can be up to two times longer than other commercially available leadless pacemakers when using the […]
Aziyo Announces FDA 510(k) Submission for CanGaroo® RM, its Next-Generation Biomaterial Envelope Enhanced with Antibiotics
SILVER SPRING, Md., April 04, 2022 (GLOBE NEWSWIRE) — Aziyo Biologics, Inc. (Nasdaq: AZYO), today announced the Company has filed a 510(k) premarket notification to the U.S. Food and Drug Administration (FDA) for CanGaroo® RM Antibacterial Envelope, its next-generation biomaterial envelope for use with implantable electronic devices (IED). Aziyo Biologics is […]