CHICAGO, Feb. 15, 2022 /PRNewswire/ — Cardiosense, Inc., a Chicago-based digital health company building a physiological waveform AI platform to manage cardiac disease, announced today that the U.S. Food and Drug Administration (FDA) has granted the company Breakthrough Device status for its novel algorithm to identify patients at risk of decompensated heart failure. The algorithm analyzes […]
Tag: FDA
Cytokinetics Announces FDA Acceptance of New Drug Application for Omecamtiv Mecarbil for the Treatment of Heart Failure With Reduced Ejection Fraction
PDUFA Target Action Date Set for November 30, 2022 FDA is Currently Not Planning to Hold an Advisory Committee Meeting to Discuss the Application SOUTH SAN FRANCISCO, Calif., Feb. 04, 2022 (GLOBE NEWSWIRE) — Cytokinetics, Incorporated (Nasdaq: CYTK) today announced that the U.S. Food & Drug Administration (FDA) has accepted and […]
BioCardia Receives FDA Breakthrough Device Designation for CardiAMP Cell Therapy System for Heart Failure
SUNNYVALE, Calif.–(BUSINESS WIRE)–BioCardia®, Inc. (Nasdaq: BCDA), a developer of cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary diseases, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the CardiAMP® Cell Therapy System for the treatment of heart failure. It is believed […]
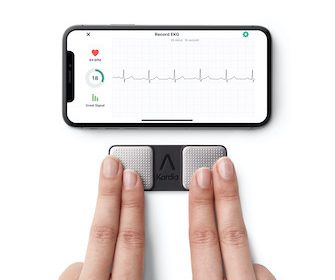
FDA Clears World’s First Credit-Card-Sized Personal ECG
AliveCor launches KardiaMobile Card, the only personal ECG slim enough to fit in a wallet for instant feedback on heart health anytime, anywhere MOUNTAIN VIEW, Calif., Feb. 1, 2022 /PRNewswire/ — AliveCor, the leading innovator in FDA-cleared personal electrocardiogram (ECG) technology, today announced the launch of KardiaMobile Card, the slimmest, most convenient personal […]
FDA approves Novartis Leqvio® (inclisiran), first-in-class siRNA to lower cholesterol and keep it low with two doses a year
– With two maintenance doses a year, Leqvio is the first and only FDA-approved small interfering RNA (siRNA) therapy for LDL-C (bad cholesterol) reduction(1) – Leqvio provides effective and sustained LDL-C reduction of up to 52% vs. placebo for certain people with atherosclerotic cardiovascular disease (ASCVD) on maximally tolerated statin […]
FDA Grants Marketing Authorization for Inferior Vena Cava Filter Removal Device
SILVER SPRING, Md., Dec. 21, 2021 /PRNewswire/ — Today, the U.S. Food and Drug Administration authorized marketing of the first laser-based device for the removal of Inferior Vena Cava (IVC) filters. The device is designed for patients who have an IVC filter, a small cage-like device inserted into the largest vein in […]
FDA Approves Two New Indications for XARELTO® (rivaroxaban) to Help Prevent and Treat Blood Clots in Pediatric Patients
XARELTO® is available as both an oral tablet and oral suspension formulation for use in appropriate children less than 18 years of age Convenient oral suspension formulation advances standard of care for children; alleviates administration challenges found with injectable alternatives XARELTO® now has 11 indications, the most of any direct […]
Mezzion Pharma Announces Positive Data Presented to FDA in Type C Meeting
SEOUL, South Korea and BETHESDA, Md., Dec. 21, 2021 /PRNewswire/ — Mezzion Pharma Co., Ltd. (Mezzion Pharma) announces that new data from its Phase 3 efficacy and safety trial (FUEL Trial) and its open label extension trial (FUEL OLE Trial) that concerns the treatment of a very large subgroup of patients with congenital single ventricle […]
HeartFlow Files for FDA Clearance of Next Generation Product Offering to Help Evaluate the Presence of Narrowings and Plaque in the Coronary Arteries
Advanced innovations leverage HeartFlow’s core AI technology and are critical additions to HeartFlow’s product portfolio for delivering complete cardiac care REDWOOD CITY, Calif., Dec. 13, 2021 (GLOBE NEWSWIRE) — HeartFlow, Inc., the leader in revolutionizing precision heart care, today announced it has submitted a 510k premarket application to the U.S. Food […]
Cardiologs’ AI Receives 510(k) Clearance for Pediatric Use
New data shows improved deep learning algorithm performs equally across all age groups, enabling more patients to benefit from the company’s cardiac diagnostics AI platform PARIS and BOSTON, Nov. 22, 2021 /PRNewswire/ — Cardiologs announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to use its AI-powered cardiac diagnostics platform […]