New indication expands addressable market for Pantheris REDWOOD CITY, CA / ACCESSWIRE / November 17, 2021 / Avinger, Inc. (NASDAQ:AVGR), a commercial-stage medical device company marketing the first and only intravascular image-guided, catheter-based system for diagnosis and treatment of Peripheral Artery Disease (PAD), today announced that it has received 510(k) clearance […]
Tag: FDA
Bristol Myers Squibb Announces New PDUFA Date for Mavacamten
PRINCETON, N.J.–(BUSINESS WIRE)–Bristol Myers Squibb (NYSE: BMY) today announced that the U.S. Food and Drug Administration (FDA) has extended the review of the New Drug Application (NDA) for mavacamten for the treatment of patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM) to April 28, 2022. The FDA notified Bristol Myers Squibb on […]
US FDA accepts supplemental New Drug Application and grants Priority Review for Jardiance® for adults with heart failure independent of left ventricular ejection fraction
If approved, Jardiance would be the first clinically proven treatment for adults across the full spectrum of heart failure regardless of ejection fraction RIDGEFIELD, Conn. and INDIANAPOLIS, Nov. 11, 2021 /PRNewswire/ — The U.S. Food and Drug Administration (FDA) has accepted a supplemental New Drug Application (sNDA) and granted Priority Review for Jardiance® (empagliflozin) 10 […]
HeartVista Receives FDA 510(k) Clearance to Deliver One Click MRI™ on Siemens Healthineers MRI Scanners
– FDA 510(k) Clearance Creates Multi-Vendor, One Click MRI™ Automation Platform to Increase Real-Time MRI Imaging Access and Accuracy for Patients – New AHA and ACC Guidelines, Making Cardiac MRI a Class I Recommendation for Chest Pain, Will Increase CMR Use to Widely Impact the Practice of Cardiology PALO ALTO, Calif.–(BUSINESS WIRE)–HeartVista, […]
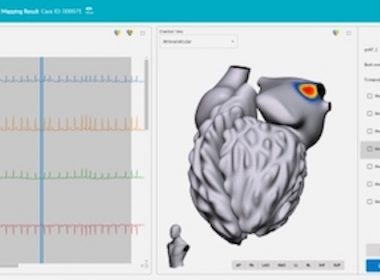
Vektor Medical Receives FDA Clearance for vMap™, First Technology Designed to Identify Arrhythmia Hot Spots Anywhere in Heart in Minutes Using Only ECG Data
Non-Invasive Technology Requires No Vests, CT or MRI Imaging, or Invasive Mapping SAN DIEGO–(BUSINESS WIRE)–Vektor Medical, Inc. today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for its novel computational ECG mapping system, vMap™. vMap is designed to map potential arrhythmia sources (hot spots) associated with stable or unstable […]
FDA Clears 12 New XO Cross Microcatheters
12 new hydrophilic-coated micro and support catheters cleared by FDA for broad use in the peripheral vasculature. PARK CITY, Utah, Nov. 2, 2021 /PRNewswire/ — Transit Scientific announced the FDA clearance of new hydrophilic-coated XO Cross Microcatheters for guidewire support, exchange, and contrast media injection in the peripheral vasculature. “The XO Cross devices […]
IMPULSE DYNAMICS RECEIVES FDA APPROVAL TO MODIFY LABELING FOR OPTIMIZER SMART MEDICAL DEVICES
FDA Authorizes Removal of “NSR” From Indications for Use MARLTON, N.J., Oct. 26, 2021 (GLOBE NEWSWIRE) — Impulse Dynamics, a global medical device company dedicated to improving the lives of people with heart failure (HF), today announced that the U.S. Food and Drug Administration approved a modification of labeling for […]
Withings Announces the FDA Clearance of ScanWatch — Its Most Medically Advanced Hybrid Smartwatch
ScanWatch becomes the first wearable device to receive FDA clearance for both atrial fibrillation detection through medical grade ECG and measuring SpO2 levels from the wrist ISSY-LES-MOULINEAUX, France, Oct. 12, 2021 /PRNewswire/ — Today, Withings, pioneers of the connected health revolution, is pleased to announce ScanWatch, its globally acclaimed and most medically advanced wearable, […]
Venclose MAVEN Earns FDA Clearance to Treat Incompetent Perforator Veins
Next-generation radiofrequency catheter offers first thermal ablation perforator treatment advancement in over 10 years SAN JOSE, Calif., Oct. 7, 2021 /PRNewswire/ — Venclose Inc., a privately-held Silicon Valley medical device company focused on innovative treatment procedures for venous reflux disease today announced FDA 510(k) clearance for Venclose Maven™, a novel radiofrequency ablation […]
Sequana Medical announces FDA approval to expand patient enrolment in North American pivotal alfapump® study (POSEIDON)
Completion of patient enrolment expected before end of year Primary endpoint read-out due in Q4 2022 59 patients already recruited in the Pivotal Cohort Ghent, Belgium – 4 October 2021 – Sequana Medical NV (Euronext Brussels: SEQUA, the “Company”), an innovator in the treatment of diuretic-resistant fluid overload in liver […]