CARMAT has responded to all remaining questions from the conditional approval Number of subjects to be enrolled in the study extended to 10 patients PARIS–(BUSINESS WIRE)–Regulatory News: CARMAT (Paris:ALCAR) (FR0010907956, ALCAR), the designer and developer of the world’s most advanced total artificial heart, aiming to provide a therapeutic alternative for […]
Tag: FDA
Abbott’s In-Development Fully Implantable Heart Pump System Earns FDA’s Breakthrough Device Designation
ABBOTT PARK, Ill., Feb. 4, 2020 /PRNewswire/ — Abbott (NYSE: ABT) today announced that the company has received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for its in-development Fully Implantable Left Ventricular Assist System (FILVAS). The FDA launched the Breakthrough Devices Program in 2018 to help expedite the development and review […]
ABBOTT ANNOUNCES FIRST-OF-ITS-KIND TRIAL TO ASSESS NEW THERAPY OPTION FOR PEOPLE AT RISK OF STROKE
– The CATALYST trial will examine Abbott’s Amplatzer™ Amulet™ device compared to non-vitamin K oral anticoagulants, the current standard in attempting to lower stroke and bleeding risks for patients with atrial fibrillation ABBOTT PARK, Ill., Feb. 3, 2020 /PRNewswire/ — Abbott (NYSE: ABT) today announced that the U.S. Food and Drug Administration […]
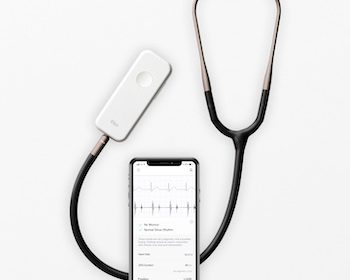
FDA Clears Eko’s AFib and Heart Murmur Detection Algorithms, Making It the First AI-Powered Stethoscope to Screen for Serious Heart Conditions
Eko’s algorithms alert clinicians to the presence of heart murmurs and atrial fibrillation (AFib) during the physical exam, converting the classic stethoscope into a powerful early detection tool SAN FRANCISCO–(BUSINESS WIRE)–Eko, a digital health company applying artificial intelligence (AI) in the fight against heart disease, announced today that the U.S. […]
Verily receives FDA 510(k) clearance for Study Watch with Irregular Pulse Monitor
One year ago, we received our first 510(k) clearance for ECG for Verily Study Watch — a wrist-worn, sensor-based device for non-invasive, continuous monitoring. Today, we’re thrilled to share that we’ve received a new 510(k) clearance for Study Watch with Irregular Pulse Monitor. This validates our approach at Verily to building robust, clinical […]
Medtronic Receives FDA Approval for Trial Evaluating New Energy Source with Pulsed Electric Fields to Treat Atrial Fibrillation
Investigative Technology Designed to Interrupt Irregular Pathways in the Heart DUBLIN, Jan. 23, 2020 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced that it received approval from the U.S. Food and Drug Administration (FDA) to proceed with an investigational device exemption (IDE) trial to evaluate the safety and effectiveness of the PulseSelect™ Pulsed Field […]
Teleflex Receives FDA Clearance for Wattson™ Temporary Pacing Guidewire
WAYNE, Pa., Jan. 22, 2020 (GLOBE NEWSWIRE) — Teleflex Incorporated (NYSE: TFX), a leading global provider of medical technologies for critical care and surgery, today announced that it received 510(k) clearance from the U.S. Food and Drug Administration for the WattsonTM Temporary Pacing Guidewire – the first commercially available bipolar temporary […]
FDA Approves Medtronic Micra™ AV, the World’s Smallest Pacemaker Which Can Now Treat AV Block
With FDA Approval, More Patients in the U.S. Are Now Candidates for a Leadless Pacing Option DUBLIN, Jan. 21, 2020 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced it has received U.S. Food and Drug Administration (FDA) approval of Micra™ AV, the world’s smallest pacemaker with atrioventricular (AV) synchrony. Micra AV is […]
Ra Medical Systems Receives FDA IDE Approval to Begin Pivotal Atherectomy Clinical Study
CARLSBAD, Calif.–(BUSINESS WIRE)–Ra Medical Systems, Inc. (NYSE: RMED), a medical device company focused on commercializing excimer laser systems to treat vascular and dermatological diseases, announces approval from the U.S. Food and Drug Administration (FDA) that the Company has provided sufficient data to support initiating an investigational device exemption (IDE) to […]
Biocardia Announces FDA Clearance for Morph DNA Deflectable Guide Catheter
SAN CARLOS, Calif., Jan. 14, 2020 (GLOBE NEWSWIRE) — BioCardia®, Inc. [OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the Morph® DNA deflectable guide catheter used to guide the Helix™ Biotherapeutic Delivery System […]