NANJING, China, Dec. 16, 2019 /PRNewswire/ — TransThera Biosciences Co. Ltd, a clinical-stage biotechnology company based in Nanjing, China, announced today that the U.S. Food and Drug Administration (FDA) has granted the company’s Investigational New Drug (IND) application for TT-00920, a novel small molecule inhibitor of Phosphodiesterase 9 (PDE 9) for the treatment of chronic heart failure. […]
Tag: FDA
First Patient Enrolls in STEMI DTU Randomized Controlled FDA Trial; Study Aims to Further Demonstrate Impella’s Safety and Effectiveness
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces initiation of the ST-Elevation Myocardial Infarction Door-to-Unloading (STEMI DTU) Pivotal Randomized Controlled Trial (RCT), which will explore whether unloading the heart’s left ventricle for 30 minutes with an Impella heart pump prior to opening blocked arteries will reduce infarct size after a heart attack and lead to […]
Bracco Diagnostics Inc.’s LUMASON® (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use, receives U.S. Food and Drug Administration approval for use in echocardiography to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border in pediatric patients with suboptimal echocardiograms
MONROE TOWNSHIP, N.J., Dec. 2, 2019 /PRNewswire/ — Bracco Diagnostics Inc., the U.S. subsidiary of Bracco Imaging S.p.A., one of the world’s leading companies in the diagnostic imaging business, announced today that its contrast agent LUMASON is the first ultrasound enhancing agent (UEA) to obtain U.S. Food and Drug Administration (FDA) approval for […]
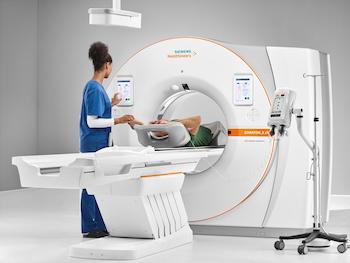
FDA Clears Siemens Healthineers SOMATOM X.cite CT Scanner With Intelligent User Interface Concept
myExam Companion intelligent user interface concept guides technologists through complex clinical tasks myExam Companion automatically adapts scans based on A.I.-trained clinical decision trees to simplify image acquisition parameters and reconstruction tasks SOMATOM X.cite improves patient comfort with an 82cm gantry bore and delivers powerful performance with Vectron tube MALVERN, Pa.–(BUSINESS […]
Medtronic Drug-Coated Balloon Receives U.S. FDA Approval to Treat Arteriovenous Fistula Lesions
Clinical Data Demonstrates IN.PACT™ AV DCB Is Safe, Reduces Reinterventions, and Maintains Access for End-Stage Renal Disease Patients Undergoing Dialysis DUBLIN, Nov. 21, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced U.S. Food and Drug Administration (FDA) approval of the IN.PACT™ AV drug-coated balloon (DCB), a paclitaxel-coated balloon indicated for the […]
Merit Medical Receives FDA Breakthrough Device Designation for WRAPSODY™ Endovascular Stent Graft System
SOUTH JORDAN, Utah, Nov. 21, 2019 (GLOBE NEWSWIRE) — Merit Medical Systems, Inc. (NASDAQ: MMSI), a leading manufacturer and marketer of proprietary disposable devices used in interventional, diagnostic and therapeutic procedures, particularly in cardiology, radiology, oncology, critical care and endoscopy, announced today that it has been granted Breakthrough Device Designation […]
FDA Grants Breakthrough Device Designation Status for BioVentrix Revivent TC Transcatheter Ventricular Enhancement System for Heart Failure
SAN RAMON, Calif.–(BUSINESS WIRE)–BioVentrix, Inc., a privately-held company with a first-in-class, transcatheter-based structural heart device to treat heart failure, today announced that the U.S. Food and Drug Administration (FDA) has granted the company Breakthrough Device Designation status for its Revivent TC™ Transcatheter Ventricular Enhancement System for heart failure. Less Invasive […]
Amarin Announced FDA Advisory Committee Voted Unanimously (16-0) to Recommend Approval of Vascepa® (icosapent ethyl) Capsules Label Expansion to Reduce Cardiovascular Risk Based on Landmark REDUCE-IT® Outcomes Trial
Cardiovascular disease events like heart attacks, stroke and death affect millions of patients in the United States and are estimated to cost $500 billion annually Millions of high-risk patients with cardiovascular disease could benefit from this cost-effective therapy if expanded label receives FDA approval; PDUFA date is December 28 DUBLIN, […]
HeartVista Receives FDA 510(k) Clearance for One Click™ Cardiac MRI Package, the First AI-assisted Cardiac MRI Scan Solution
LOS ALTOS, Calif.–(BUSINESS WIRE)–HeartVista, a pioneer in AI-assisted MRI solutions, today announced that it received 510(k) clearance from the U.S. Food and Drug Administration to deliver its AI-assisted One Click™ MRI acquisition software for cardiac exams. Despite the many advantages of cardiac MRI, or cardiac magnetic resonance (CMR), its use […]
Endologix, Inc. Comments on Recent FDA Update on Type III Endoleaks with AFX® Endovascular AAA System
IRVINE, Calif.–(BUSINESS WIRE)–Endologix, Inc. (Nasdaq: ELGX), a developer and marketer of innovative treatments for aortic disorders, today announced a response to the U.S. Food and Drug Administration (FDA) update regarding Type III endoleaks with the AFX Endovascular AAA System. Dr. Matt Thompson, Chief Medical Officer of Endologix Inc. commented, “On […]