Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced that the Center for Devices and Radiological Health (CDRH) of the Food and Drug Administration (FDA) has approved the company’s premarket approval (PMA) application for its HeartStart OnSite defibrillator [1] and HeartStart Home defibrillator [2], […]
Tag: FDA
Canon Medical Receives FDA Clearance for Deep Convolutional Neural Network Image Reconstruction Technology for CT
TUSTIN, Calif.–(BUSINESS WIRE)–Building on its advanced image reconstruction technologies, Canon Medical Systems USA, Inc. has received 510(k) clearance on its new deep convolutional neural network (DCNN) image reconstruction technology, ushering in a new era for CT. Canon Medical’s Advanced Intelligent Clear-IQ Engine (AiCE) uses a deep learning algorithm to differentiate signal from […]
Materialise Receives FDA Clearance for Cardiovascular Planning Software Suite
CHICAGO–(BUSINESS WIRE)–TVT2019 – Structural Heart Summit — Materialise (NASDAQ:MTLS), a global 3D printing software and solutions company, has received FDA clearance for its Mimics Enlight cardiovascular planning software suite. The first release will support clinicians planning complex transcatheter mitral valve replacement (TMVR) procedures. Mimics Enlight is based on the strengths of […]
GORE® CARDIOFORM ASD Occluder Receives FDA Approval for the Treatment of Atrial Septal Defects
FLAGSTAFF, Ariz., June 4, 2019 /PRNewswire/ — W. L. Gore & Associates (Gore) announced the U.S. Food and Drug Administration’s (FDA’s) premarket approval (PMA) of the GORE® CARDIOFORM ASD Occluder for the percutaneous closure of ostium secundum atrial septal defects (ASDs). The FDA approval was supported by data collected from the pivotal stage of the Gore […]
Medtronic Gains U.S. FDA Clearance for New Catheter System for HIS Bundle Pacing
DUBLIN – May 29, 2019 – Medtronic plc (NYSE:MDT) today announced U.S. Food and Drug Administration (FDA) clearance and commercial launch for the SelectSite(TM) C304-HIS deflectable catheter system for use in procedures involving His-bundle pacing (HBP). The SelectSite C304-HIS deflectable catheter system features a deflectable, out-of-plane curve to reach the bundle […]
Gore Receives FDA Approval for the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) today announced that the U.S. Food and Drug Administration (FDA) has granted regulatory approval for commercial distribution for the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System, a unique Thoracic Endovascular Aortic Repair (TEVAR) solution combining new levels of control […]
FDA grants clearance for a heart murmur detection solution on a personal mobile device
eMurmur ID is an artificial intelligence-based solution that enables healthcare providers to detect and classify heart murmurs with expert-level accuracy OTTAWA, May 14, 2019 /PRNewswire/ – eMurmur® today announced that its flagship “eMurmur ID” solution has received FDA clearance, enabling a new era in heart murmur detection. eMurmur ID is a mobile and cloud […]
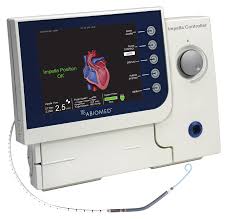
FDA Approves Impella 5.0 and Impella LD Extended Duration of Use to 14 Days for Cardiogenic Shock Derived from AMI or Cardiomyopathy
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD) announces the U.S. FDA has approved the expansion of the Impella 5.0 and Impella LD PMA labeling for the treatment of cardiogenic shock. The expansion extends the duration of support for each pump from 6 days to 14 days. The Impella 5.0 and the Impella LD are forward flow heart pumps […]
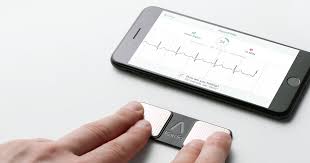
FDA Grants First Ever Clearance For Six-Lead Personal ECG Device
MOUNTAIN VIEW, Calif., May 13, 2019 /PRNewswire/ — AliveCor, the leader in FDA-cleared consumer electrocardiogram technology (ECG), today announced its third FDA clearance in three months, making KardiaMobile 6L the world’s first available six-lead personal ECG device. This highly anticipated clearance gives patients and their physicians an even more detailed view into patients’ […]
BioCardia Receives FDA Clearance for AVANCE Steerable Introducer for Transseptal Access to Heart
SAN CARLOS, Calif., May 08, 2019 (GLOBE NEWSWIRE) — BioCardia®, Inc. [OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the AVANCE™ steerable introducer product family, designed for introducing various cardiovascular catheters into the heart, including […]