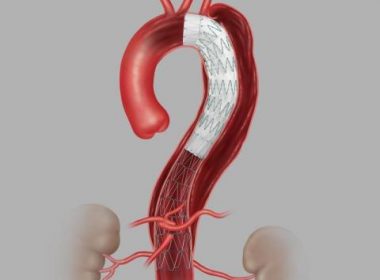
BLOOMINGTON, Ind.–(BUSINESS WIRE)–Today, Cook Medical announced its recent approval from the U.S. FDA for its Zenith Dissection Endovascular System. The system, consisting of a proximal stent-graft component and a distal bare stent component, provides physicians a less invasive alternative to open surgery for repair of Type B dissections of the […]
Tag: FDA
XableCath Crossing Catheters FDA Cleared
SALT LAKE CITY, Jan. 30, 2019 (GLOBE NEWSWIRE) — XableCath, Inc., announced that its XableCath™ blunt and abrasion tip catheters, were cleared by the FDA as Peripheral Crossing Catheters. The catheters have been safely and successfully used to cross challenging lesions in both arterial CTO’s and chronic obstructive venous lesions. […]
CorMatrix® Cardiovascular, Inc. receives FDA IDE approval to conduct the safety and feasibility clinical trial of the Cor™ TRICUSPID ECM® valve for pediatric and adult patients
ATLANTA, Jan. 24, 2019 /PRNewswire/ — CorMatrix® Cardiovascular, Inc. www.cormatrix.com, a leading developer of regenerative cardiovascular medical devices, today announced FDA approval of an early feasibility study IDE to evaluate the safety and feasibility of the Cor™ TRICUSPID ECM® cardiac valve for adults with endocarditis and for pediatric patients with congenital heart valve disease. The […]
Rebound Therapeutics Announces FDA Clearance of the Aurora Surgiscope System for Minimally Invasive Neurosurgery
IRVINE, Calif.–(BUSINESS WIRE)–Rebound Therapeutics® Corporation today announced FDA 510k clearance of their AURORA Surgiscope® System, the world’s first single-use disposable Neurosurgical Endoscope for minimally invasive access, visualization and illumination of the target neuro anatomy. The Aurora Surgiscope System consists of two components: the sterile, single use, neurological endoscope called the Aurora Surgiscope […]
Imperative Care Announces FDA Clearance of Initial Products
CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc., a company singularly dedicated to finding answers to unsolved problems in stroke, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its first family of access catheters, designed to deliver interventional treatments during minimally invasive neurovascular procedures for aneurysms, stroke and other brain […]
Verily Study Watch Receives FDA 510(k) Clearance for ECG
In April of 2017, we launched Verily Study Watch, an investigational device for capturing health information from clinical research participants while serving as an easy-to-read watch for daily wear. Since then, Study Watch has been used by thousands of participants in clinical research studies run by Verily and through our partners, such […]
FDA Additionally Clears RAPID™ Imaging Platform for Use in Selecting Acute Stroke Patients for Clot Removal
MENLO PARK, Calif.–(BUSINESS WIRE)–iSchemaView, the worldwide leader in advanced imaging for stroke, today announced that the FDA has cleared the RAPID neuroimaging platform for use in selecting stroke patients who are likely to benefit from endovascular thrombectomy (clot removal). Specifically, this additional clearance means that RAPID CT-Perfusion and RAPID MR-Perfusion […]
FDA Approves World’s First Device for Treatment of Premature Babies and Newborns with an Opening in Their Hearts (a Common Congenital Defect)
ABBOTT PARK, Ill., Jan. 14, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced the U.S. Food and Drug Administration (FDA) approved the Amplatzer Piccolo™ Occluder, the world’s first medical device that can be implanted in the tiniest babies (weighing as little as two pounds) using a minimally invasive procedure to treat patent ductus arteriosus, or […]
MRI Interventions Receives FDA Clearance for ClearPoint® PURSUIT™ Neuro Aspiration System
IRVINE, Calif., Jan. 03, 2019 (GLOBE NEWSWIRE) — MRI Interventions, Inc. (OTCQB:MRIC), a platform neurosurgery company with products designed for navigation in procedures involving deep-brain stimulation, ablation, and gene and drug delivery today announced FDA Clearance of the ClearPoint PURSUIT Neuro Aspiration system. The PURSUIT device was designed in collaboration […]
ARCA biopharma Updates Special Protocol Assessment Request to FDA for Gencaro Phase 3 Atrial Fibrillation Clinical Trial
WESTMINSTER, Colo., Dec. 20, 2018 (GLOBE NEWSWIRE) — ARCA biopharma, Inc. (Nasdaq: ABIO), a biopharmaceutical company applying a precision medicine approach to developing genetically-targeted therapies for cardiovascular diseases, today announced that it has submitted an amendment to its Special Protocol Assessment (SPA)request to the U.S. Food and Drug Administration (FDA). The amendment addresses […]