WALTHAM, Mass.–(BUSINESS WIRE)–Thermedical® announced today that the U.S. Food and Drug Administration (FDA) has approved its Investigational Device Exemption (IDE) application for the Early Feasibility Study (EFS) of the groundbreaking Durablate™ ablation catheter. The single-arm, observational study is designed to evaluate the safety and effectiveness of the Durablate catheter to […]
Tag: FDA
Integer Receives FDA Approval for Thin Wall Radial Introducer Kit, RadialSeal™
PLYMOUTH, Minn., July 31, 2018 (GLOBE NEWSWIRE) — Integer Holdings Corporation (“Integer”) (NYSE:ITGR), a leading medical device outsource manufacturer, announced today that it has received FDA and CE Mark approval for their new radial access introducer, RadialSeal™. The RadialSeal Introducer Kit is founded on Integer’s core technology platforms in guidewires and […]
LifeSignals Announces FDA Clearance of Health Care Vital Sign Wireless Monitoring Patch and ECG Application
FREMONT, Calif.–(BUSINESS WIRE)–LifeSignals announced today that it received FDA clearance for its wireless LP1100 Life Signal Patch for enabling the next generation of wearable, healthcare monitoring devices. It is built on two solid technology foundations to provide unprecedented attributes unachieved by another ECG patch product to date. It deploys the […]
Zebra Medical Vision Announces FDA 510(k) Clearance of Its Coronary Calcium Algorithm
SHEFAYIM, Israel–(BUSINESS WIRE)–Zebra Medical Vision (http://zebra-med.com/) announces today it has received 510(k) clearance for its Coronary Calcium Scoring algorithm. The algorithm, capable of automatically calculating a patient’s Agatston equivalent coronary calcium score from ECG gated CT scan, provides physicians with important data used in the assessment of the risk for coronary […]
Medtronic Receives FDA Approval for Less-Invasive Heart Pump Implant Procedure
DUBLIN – July 11, 2018 – Medtronic plc (NYSE: MDT) has received United States Food and Drug Administration (FDA) approval for a less-invasive implant approach of its HVAD(TM) System, a left ventricular assist device (LVAD) for patients with advanced heart failure. The HVAD System is the smallest commercially available LVAD, and […]
Beckman Coulter Diagnostics Receives U.S. FDA 510(k) Clearance for High- sensitivity Access hsTnI Assay
BREA, Calif., June 27, 2018 /PRNewswire/ — Beckman Coulter Diagnostics announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its new high-sensitivity troponin (hsTnI) assay, Access hsTnI, for use on the Access 2, DxI and the entire Access family of immunoassay systems. The Access hsTnI assay […]
Avenu Medical Receives FDA Approval for Ellipsys Vascular Access System for Non-Surgical Dialysis Fistula Creation
SAN JUAN CAPISTRANO, Calif.–(BUSINESS WIRE)–Avenu Medical, Inc. announced today that it has received De Novo marketing authorization from the Food and Drug Administration (FDA) to market its Ellipsys® Vascular Access System, an innovative, minimally-invasive catheter-based system designed for End-Stage Renal Disease (ESRD) patients requiring hemodialysis. The FDA’s action will provide U.S. […]
Caladrius Receives FDA Regenerative Medicine Advanced Therapy Designation for CD34+ Cell Therapy for Treating Refractory Angina
BASKING RIDGE, N.J., June 19, 2018 (GLOBE NEWSWIRE) — Caladrius Biosciences, Inc. (Nasdaq:CLBS) (“Caladrius” or the “Company”), a clinical-stage biopharmaceutical company with multiple technology platforms targeting select cardiovascular indications and autoimmune diseases, announces today that the U.S. Food and Drug Administration (“FDA”) has granted regenerative medicine advanced therapy (“RMAT”) designation to […]
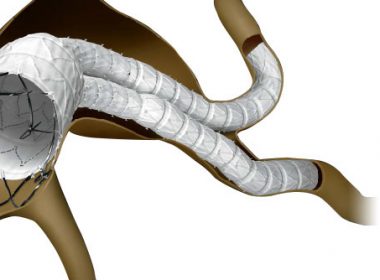
FDA Advisory Committee Votes in Favor of Cardinal Health’s INCRAFT® AAA Stent Graft System for the Endovascular Treatment of Infrarenal Abdominal Aortic Aneurysms
DUBLIN, Ohio, June 12, 2018 /PRNewswire/ — Cardinal Health (NYSE: CAH) today announced that the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee has provided a favorable recommendation on the premarket approval application for INCRAFT® AAA Stent Graft System (INCRAFT). The panel voted 11 to 4 in […]
Celixir announces US FDA approval of the IND application for cell therapy Heartcel
Stratford-upon-Avon, UK, 08 June 2018 – Celixir, a privately owned company discovering and developing life-saving advanced therapies, announces that the US Food and Drug Administration (FDA) has approved its Investigational New Drug application (IND) for HeartcelTM, its immuno-modulatory progenitor (iMP) cell therapy for the treatment of adult heart failure. Celixir announced […]