AUSTIN, Texas–(BUSINESS WIRE)–BioStable Science & Engineering, Inc. announced today it has received FDA market clearance for the HAART 200 Aortic Annuloplasty Device, the first and only annuloplasty device designed specifically for bicuspid aortic valve repair. With FDA clearance of both the HAART 300 and HAART 200 Aortic Annuloplasty Devices, BioStable […]
Tag: FDA
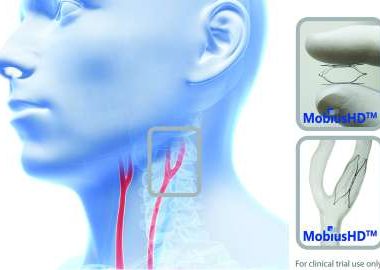
Vascular Dynamics, Inc. Announces FDA Approval To Initiate CALM 2 Trial For Mobiushd System For The Treatment Of Resistant Hypertension
MOUNTAIN VIEW, Calif., Aug. 15, 2017 (GLOBE NEWSWIRE) — Vascular Dynamics, Inc., (VDI) a privately held medical device company developing novel solutions for the treatment of hypertension, today announces that the United States Food and Drug Administration (FDA) has approved the company’s Investigational Device Exemption (IDE) application to initiate its […]
AUM CARDIOVASCULAR RECEIVES FDA CLEARANCE FOR REVOLUTIONARY DIAGNOSTIC HEART DEVICE
NORTHFIELD, MINNESOTA— AUM Cardiovascular announced that it has received clearance from the Food and Drug Administration for CADence,™ a non-invasive acoustic and ECG device designed to help physicians detect physiological and pathological heart murmurs. The reusable, non-invasive, radiation-free handheld device, which is now available in the United States, records sounds […]
Penumbra initiates recall of 3D Revascularation Device
Penumbra Inc. Recalls 3D Revascularization Device Due to Wire Material That May Break or Separate During Use Penumbra Inc. is recalling the Penumbra 3D Revascularization device because there is a risk of the delivery wire breaking or separating during use. Fractured pieces of the delivery wire could be left inside […]
Intact Vascular Release: FDA Approves 6-Month Primary Endpoint For The Tack Endovascular System In Below The Knee Disease
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced the U.S. Food and Drug Administration (FDA) approved an Investigational Device Exemption (IDE) supplemental application to modify the primary endpoint in the Tack Optimized Balloon Angioplasty II Below the Knee (TOBA II BTK) clinical trial from 12 months to […]
“Cardiologs ECG Analysis Platform” Receives FDA Clearance
PARIS–(BUSINESS WIRE)–Cardiologs Technologies SAS announced today that it has received FDA clearance of its Cardiologs ECG Analysis Platform, a cloud-based cardiac monitoring-analysis web service powered by artificial intelligence (AI). Cardiologs aids physicians in screening for atrial fibrillation (AFib) and other arrhythmias using long-term ambulatory ECG monitoring recordings. The Cardiologs system is also CE-marked […]
OrbusNeich Announces FDA Clearance, Launch of Coronary Dilatation Catheters
HONG KONG, June 6, 2017 /PRNewswire/ — Announcement marks company‘s official entry into the US market OrbusNeich, a global company specializing in the provision of life-changing vascular solutions, has announced the launch of its world-renowned Sapphire PTCA balloon dilatation catheters following their recent 510k clearance by the FDA: the Sapphire™ […]
Edwards SAPIEN 3 Valve Receives FDA Approval For Aortic, Mitral Valve-In-Valve Procedures
IRVINE, Calif., June 5, 2017 — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced it has received U.S. Food and Drug Administration (FDA) approval for aortic and mitral valve-in-valve procedures using the Edwards SAPIEN 3 transcatheter heart valve. […]
RA Medical’ DABRA Begins First Commercial, FDA-Cleared, In-Patient Use
NEW ORLEANS, La.–(BUSINESS WIRE)–Ra Medical Systems, makers of cardiovascular and dermatology catheters and excimer lasers, today announced the commercial launch in the United States of the Company’s groundbreaking DABRA™ System for the treatment of Peripheral Artery Disease. This follows FDA clearance earlier this week. Dr. Athar Ansari of the California […]
FDA Grants Market Clearance to RA Medical’s New PAD System
CARLSBAD, Calif.–(BUSINESS WIRE)–In an effort to battle Peripheral Artery Disease (PAD), the leading cause of limb amputations, the Food and Drug Administration (FDA) announced today that it has granted market clearance to Ra Medical Systems, makers of excimer lasers and catheters for cardiovascular and dermatological diseases, for the Company’s groundbreaking […]