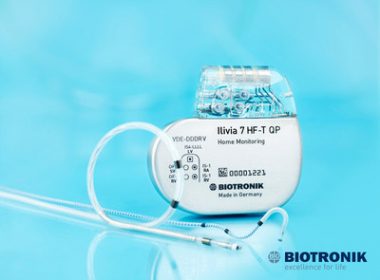
LAKE OSWEGO, Ore., May 8, 2017 /PRNewswire/ — BIOTRONIK today announced FDA approval of the company’s MultiPole Pacing (MPP) technology, providing physicians with additional treatment options for heart failure patients who have been non-responsive to cardiac resynchronization therapy (CRT).1 MPP will be available on new BIOTRONIK CRT defibrillator (CRT-D) systems for […]
Tag: FDA
Boston Scientific (BSX) Snags FDA Approval for Resonate Family of High-Voltage Devices
MARLBOROUGH, Mass., May 9, 2017 /PRNewswire/ — Boston Scientific (NYSE: BSX) has received U.S. Food and Drug Administration (FDA) approval for the Resonate family of implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy defibrillator (CRT-D) systems. The approval includes new features in the Resonate devices including SmartCRT technology with Multisite Pacing […]
Acutus Medical Snags FDA OK for AcQGuide Steerable Sheath
CARLSBAD, Calif.–(BUSINESS WIRE)–Acutus Medical®, a company committed to transforming the lives of millions of patients with complex arrhythmias, today announced the FDA clearance of the AcQGuide™ Steerable Sheath. The company will also feature several international presentations highlighting the AcQMap® High Resolution Imaging and Mapping System at the Heart Rhythm Society’s […]
Teleflex Inc. (TFX) Wins FDA 510(k) Clearance for the Arrow AC3 Optimus Intra-Aortic Balloon Pump
Teleflex Receives FDA 510(k) Clearance for the Arrow® AC3 OptimusTM Intra-Aortic Balloon Pump (IABP) Advanced IABP performance with the ability to provide optimized therapy to the most challenging patient conditions, even patients with the most severe arrhythmias and heart rates as high as 200 bpm.1 WAYNE, Pa.–(BUSINESS WIRE)–Teleflex Incorporated (NYSE: […]
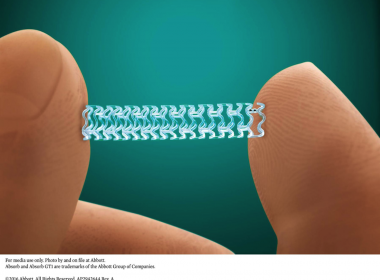
Lessons We Can Learn From the Abbott BVS Data
By Ken Dropkiewski The bioresorbable stent was approved for use by the FDA in July of 2016. The Absorb GT1 Bioresorbable Vascular Scaffold System, manufactured by Abbott, was all set to solve a litany of the problems inherent with conventional cardiac and vascular stents. Conventional stents, made of flexible metal […]
Biotricity Files for its Second and Final FDA 510(k) to Bring Bioflux Solution to Market
REDWOOD CITY, Calif., April 12, 2017 (GLOBE NEWSWIRE) — Biotricity, Inc. (OTCQB:BTCY), a medical diagnostic and consumer healthcare technology company dedicated to delivering innovative, biometric remote monitoring solutions, has filed for a second and final 510(k) for the hardware portion of its Bioflux solution with the U.S. Food and Drug Administration […]
Cardiovascular Systems Snags FDA Nod for Its Diamondback 360 Coronary Orbital Atherectomy System (OAS)
Cardiovascular Systems, Inc. Receives Approval for the Diamondback 360® Coronary Orbital Atherectomy System (OAS) Micro Crown in the United States OAS Micro Crown Approved to Treat Severely Calcified Coronary Lesions Only Atherectomy Device Designed to Both Pilot Tight Lesions and Treat Up to 4mm Vessels with a Single Device ST. […]
Humacyte Receives FDA Regenerative Medicine Advanced Therapy (RMAT) Expedited Review Designation for HUMACYL® in Vascular Access for Hemodialysis
Press Release RESEARCH TRIANGLE PARK, N.C. — Humacyte, an innovator in biotechnology and regenerative medicine, announced today that the U.S. Food and Drug Administration (FDA) has granted HUMACYL®, its investigational human acellular vessel (HAV), the Regenerative Medicine Advanced Therapy (RMAT) designation. This designation means that the FDA will help facilitate […]
Aegis Medical Innovations Announces FDA Approval of Clinical Trial
VANCOUVER, British Columbia–(BUSINESS WIRE)–Aegis Medical Innovations Inc. (Aegis) today announced that it has received Investigational Device Exemption approval from the U.S. FDA to initiate a clinical trial in the U.S. for its medical device called the Sierra Ligation System (Sierra). Aegis developed the Sierra technology in partnership with the Mayo […]
BioStable Science & Engineering Wins FDA Nod for Its HAART 300 Aortic Annuloplasty Device
BioStable Science & Engineering Announces FDA Clearance of the HAART™ 300 Aortic Annuloplasty Device AUSTIN, Texas–(BUSINESS WIRE)–BioStable Science & Engineering, Inc. announced today it has received FDA market clearance for the HAART 300 Aortic Annuloplasty Device, the first commercially available internal annuloplasty device designed for aortic valve repair. BioStable expects […]