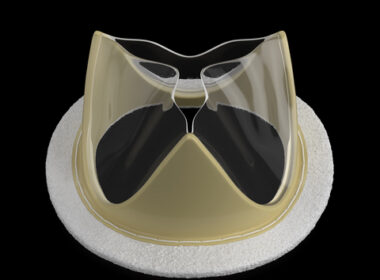
World’s First Approved Polymer Heart Valve Now Available at Select Centers GOA, India–(BUSINESS WIRE)–Foldax® Inc., a leader in the development of innovative polymer heart valves, in conjunction with its local manufacturing partner Dolphin Life Science LLP, today announced the launch of the TRIA™ Mitral Valve in India, marking the first time […]
Tag: Foldax
Foldax Secures Approval for TRIA Mitral Heart Valve in India
SALT LAKE CITY–(BUSINESS WIRE)–Foldax® Inc., a leader in heart valve innovation, today announced that the Indian Central Drugs Standard Control Organization (CDSCO) approved its TRIA™ Mitral Valve. Dolphin Life Science India LLP will locally manufacture the TRIA Mitral Valve in India. Foldax’s vision for its novel polymer heart valves is […]
Foldax® Reports Positive Clinical Results for TRIA Mitral Surgical Heart Valve Using Novel LifePolymer Material
SALT LAKE CITY–(BUSINESS WIRE)–Foldax®, Inc., a leader in the development of innovative, polymer heart valves, today announced positive 30-day results from a prospective, multicenter, single-arm, clinical study of the TRIA™ mitral surgical heart valve with LifePolymer™ conducted in India. At 30 days following surgery, TRIA demonstrated favorable safety and hemodynamics, […]
TRIA™ Polymer Surgical Valves Surpass 200 Patient Life Years
SALT LAKE CITY–(BUSINESS WIRE)–Foldax®, Inc., a leader in the development of innovative, polymer heart valves, today announced that its TRIA valves have surpassed 200 patient life years in human recipients. Patient life years represent the cumulative time the valve has been implanted in patients, offering insight into the duration of […]
Foldax, Inc. Signs Manufacturing Agreement with Dolphin Life Science India LLP to Expedite Upcoming Commercial Availability
SALT LAKE CITY–(BUSINESS WIRE)–Foldax® Inc., a pioneer in the development of innovative, polymer heart valves, today announced a manufacturing agreement with Dolphin Life Science India LLP to enable in-country manufacturing of the TRIA™ polymer mitral surgical heart valve outside of the U.S. for the first time. “We are honored to collaborate […]
First Patients Outside of U.S. Treated with TRIA Polymer Surgical Mitral Heart Valve
SALT LAKE CITY–(BUSINESS WIRE)–Foldax®, Inc., a leader in the development of innovative, polymer heart valves, today announced that the first 30 patients have been treated outside of the U.S., in the Indian clinical trial of the TRIA™ surgical mitral heart valve. The TRIA mitral valve is designed specifically to accommodate […]
Foldax, Inc. Receives Full Ownership Rights to Proprietary Polymer Patent Portfolio
SALT LAKE CITY–(BUSINESS WIRE)–Foldax® Inc. today announced that it has executed an agreement with Australia’s national science agency, CSIRO, for full ownership of a polymer technology patent family. Foldax has incorporated the polymer technology into its LifePolymer™ materials platform and has conducted testing, which indicates the materials are compatible with blood, with […]
Foldax Appoints Gregory D. Casciaro as Chief Executive Officer
SALT LAKE CITY–(BUSINESS WIRE)–Foldax®, Inc. today announced that medtech veteran Gregory D. Casciaro has been named Chief Executive Officer of the company, effective yesterday. In his more than 30 years in the medical technology arena, Mr. Casciaro has developed a track record of success leading and growing both private and […]
First Patients Outside of U.S. Treated with TRIA Biopolymer Surgical Aortic Heart Valve in India Clinical Trial
SALT LAKE CITY–(BUSINESS WIRE)–Foldax®, Inc. today announced that the first patients have been enrolled in the Drugs Controller General of India (DCGI)-approved clinical trial of the TRIA™ biopolymer surgical aortic heart valve. The TRIA valve reimagines the heart valve by combining the company’s proprietary biopolymer – LifePolymer™ – with an […]
TRIA Transcatheter Biopolymer Heart Valve Shows Promise in Pilot Animal Study
Data Presented at Cardiovascular Research Technologies (CRT) Annual Conference SALT LAKE CITY–(BUSINESS WIRE)–Foldax®, Inc. today announced the presentation of results from the first animal study of its transcatheter valve from the TRIA™ biopolymer heart valve portfolio this past weekend at the Cardiovascular Research Technologies (CRT) annual conference. The transcatheter TRIA […]